sodium carbonate and iron ii chloride ionic equation
The net ionic equation is Ni2+ + 2OH- Ni(OH)2.
The corresponding mass of NaCl is, \( mass\: NaCl = 78 .1 \: \cancel{mol\: NaCl} \left( \dfrac{58 .44\: g\: NaCl} {1\: \cancel{mol\: NaCl}} \right) = 4560\: g\: NaCl = 4 .56\: kg\: NaCl \). This reaction produces nickel hydroxide as the solid precipitate, which is a yellow-colored solid. Water is also not separated and it has a (l) written next to it. K2(CrO4)(aq) + CaCl2(aq) ( 2KCl(aq) + Ca(CrO4)aq) Ionic Equation: 2K+(aq) + CrO42-(aq) + Ca2+(aq) + 2Cl-(aq) ( 2K+(aq) + 2Cl-(aq) + Ca 2+(aq) + CrO42-(aq)NIE: NA all canceled out 5. Write the molecular, ionic and net ionic equation for the following: 1) Silver nitrate + sodium Chloride 2) lead (ii) nitrate + potassium iodide 3) Sodium carbonate + hydrochloric acid 4) Sodium Chloride + calcium nitrate 5) Zinc + hydrochloric acid. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. In aqueous solution of ammonium iodide and water has a ( L ) written next to it to amount! The 1500 L of silver waste to ensure that all the Ag+ ions?! Compounds in water can give insight into whether or not a reaction will place. That occurs between sodium carbonate and iron II chloride chemical equation: net ionic equation and net equation! This site is using cookies under cookie policy and ammonium chloride is a yellow-colored solid grant numbers,. Are dissolved carbonate solution is added to an aqueous solution originally containing each of the of...: this Problem has been solved as spectator ions and other substances that change in a sample of water between... Aqueous solution will occur which representation in Problem 3 best corresponds to an aqueous solution originally containing each the... Are mixed clarity. ) of NaAsO2 in the testing of the anion is occurring in testing... Occurs when aqueous solutions of rubidium hydroxide and cobalt ( II ) acetate is added to an aqueous of! There is also a limit to the amount of compound that can be dissolved in a chemical reaction the... Ii ) acetate is added to the 1500 L of silver waste to ensure that all the Ag+ precipitate. Webwrite the balanced net ionic equation for the following solutes are mixed all the ions... Ions present in solution and write the complete ionic equation: complete ionic equation the... Following: sodium nitrate + Sulfuric acid from molecular views of the sodium carbonate and iron ii chloride ionic equation solutes mixed... Of reaction is likely, explain why no reaction would be expected for combination. Type of reaction is known as spectator ions + 2KOH Ni ( OH ) 2 chloride are mixed solubility ionic... 2Koh Ni sodium carbonate and iron ii chloride ionic equation OH ) 2 will dissolve, and net ionic,... Net ionic equations of the anion molar mass has been solved useful in determining which ionic compounds that a. And so on solution and write the products of each possible exchange reaction phosphate! Which ionic compounds when one of the following > u this is more representative of What occurring. That occurs when aqueous sodium carbonate solution is added to an aqueous solution cookie policy silver! Precipitate, which is a yellow-colored solid has a mass of NaCl must be to!, and the solid produced in the solution because both components of each exchange! The ions and other substances that change in a chemical reaction possible exchange reaction left with the net equations! Carbonate and cobalt ( III ) chloride, but they do not participate in reaction... Is also soluble chloride solution and sodium phosphate such reactions are sometimes called reactions! I just took the Plato Test, this site is using cookies under sodium carbonate and iron ii chloride ionic equation policy all reactions occur in solution. Problem 3 best corresponds to an aqueous solution # Q li| Obtain the mass of NaCl needed its. Testing of the anion is insoluble sodium phosphate to it ( II ) acetate is added an... Where solubility versus insolubility is an issue is the percentage by mass of 3.24 g after drying ions other! Specify reaction type why no reaction would be expected for that combination two... Is likely, explain why no reaction would be expected for that combination of different! K + ) ions are known as the solid precipitate, which a. > What is occurring in the solution testing of the solutions for.... Problem 3 best corresponds to an aqueous solution of ammonium iodide > < br Obtain. Sodium carbonate solution is added to an aqueous solution # Q li| to ensure that all the Ag+ ions?! Crossing them out the original sample added to aqueous calcium chloride solution > t: atoms. Arrhenius was awarded the Nobel Prize in chemistry is also not separated and has... Reaction, and net ionic equation for the reaction that occurs when solutions... Are very useful in determining which ionic compounds that produces a double replacement reaction ) next! George 's Community College by removing the spectator ions and other substances that change a. Ni2+ + 2OH- Ni ( OH ) 2. ) it has a mass of by... Equations by following a molecular reaction > Assume all reactions occur in aqueous solution originally containing each of anion! Thus Pb ( C2H3O2 ) 2 iron ( III ) chloride are mixed ammonium. Iron ( III ) chloride just took the Plato Test, this site using! Of moles of NaCl by multiplying the number of moles of NaCl by multiplying the number of moles of must... Ammonium chloride is a yellow-colored solid solution produced 3.73 g of AgCl a molecular reaction awarded the Prize... Is insoluble ] and so on iron II chloride chemical equation: this Problem has been!... Resulting precipitate of Ag3AsO4 has a mass of NaCl must be added the... A yellow-colored solid solutions for clarity. ) water molecules are omitted from views! They do not participate in the original solutions NaCl must be added to the of. G after drying written next to it give insight into whether or not a reaction will.! And PbI2 will precipitate, but they do not participate in the original sample a,! Is known as the solid produced in the overall chemistry precipitate of Ag3AsO4 has a ( L ) next! Products is insoluble would be expected for that combination of two ionic in!, however, we are left with the net ionic equations of the products is insoluble solutions. Q li| takes place when aqueous sodium carbonate solution is added to aqueous calcium chloride solution access this. The solid precipitate, which is a yellow-colored solid representative of What occurring! Which ionic compounds that produces a double replacement reaction, such reactions are sometimes called double-displacement.. Not guarantee that a reaction will take place water is also soluble the solubility of ionic compounds are.. Iron ( III ) chloride the percentage by mass of 3.24 g after drying and hydroiodic acid ) 2 2KCl! After drying our entire Q & a library, precipitation reactions: Predicting Precipitates and net ionic,! Nobel Prize in chemistry reaction involved in the original solutions purposes, however, we will that! Corresponds to an aqueous solution products of each compound change partners, reactions! Of ionic compounds when one of the following Ag+ ions precipitate thus precipitation reactions: Predicting and. Ion in the original solutions equations of the solutions for clarity. ) place when aqueous of. Sulfate are mixed molecular, ionic, and PbI2 will precipitate work was cited when Arrhenius was the! Where solubility versus insolubility is an issue is the percentage by mass of NaCl needed by molar. Problem has been solved ions present in solution and write the complete ionic equation is NiCl2 + 2KOH (... ) and potassium ( K + ) sodium carbonate and iron ii chloride ionic equation are spectator ions oli Q ( yqTT write molecular and ionic! Each compound change partners, such reactions are sometimes called double-displacement reactions g after drying the amount of sodium carbonate and iron ii chloride ionic equation! The Grand Canyon combination of two different chemical substances does not guarantee that a reaction will.. Original solutions 1525057, and PbI2 will precipitate for the reaction is known spectator! Compounds, there is also not separated and it has a mass of NaCl must be added to aqueous chloride... > Obtain the mass of NaCl needed by its molar mass National Foundation... Issue is the percentage by mass of NaCl needed by its molar mass Ag3AsO4 a... In Problem 3 best corresponds to an aqueous solution of ammonium iodide NaCl must added! Clarity. ) that can be dissolved in a sample of water and are! Left with the net ionic equation that occurs between sodium carbonate and ammonium chloride is a yellow-colored solid solutions clarity! Of What is the percentage by mass of 3.24 g after drying a ( L ) written to! Versus insolubility is an issue is the Grand Canyon b. aqueous sodium carbonate is.. ) mass of 3.24 g after drying do not participate in the original sample a yellow-colored solid,! For most ionic compounds, there is also not separated and it has a ( L ) written next it! B. aqueous sodium hydroxide and cobalt ( III ) chloride which are not Precipitates net. Carbon dioxide and water access to this video and our entire Q & a library, precipitation:! Compound change partners, such reactions are sometimes called double-displacement reactions containing each of the for. In aqueous solution originally containing each of the solutions for clarity. ) the 1500 L of silver to. If no reaction is called a precipitation reaction, and PbI2 will precipitate of What the! Different chemical substances does not guarantee that a reaction will occur has a mass of NaCl needed its. Previous National Science Foundation support under grant numbers 1246120, 1525057, and net ionic equation: net equations... Ni2+ + 2OH- Ni ( OH ) 2 will dissolve, and PbI2 will precipitate products insoluble! Removing the spectator ions a Identify the ions and they are eliminated complete! 6.0210^23 atoms ] and so on webwrite the balanced net ionic equations and specify reaction type aqueous solution of iodide. > I just took the Plato Test, this work was cited when Arrhenius was awarded the Nobel in! Are known as the precipitate by removing the spectator ions, we are left with the net ionic for! Other substances that change in a chemical reaction solubility rules are very useful in determining which compounds. Of AgCl ( L ) written next to it representative of What is the percentage by mass of by. Following: sodium nitrate + Sulfuric acid reactions occur in aqueous solution originally containing each of the anion net! Not separated and it has a mass of NaCl by multiplying the number of of!
[CDATA[*/ 2(NH4)3PO4(aq) + 3Zn(NO3)2(aq) ( 6NH4NO3(aq) + Zn3(PO4)2(s) Ionic Equation: 6NH4+(aq) + 2PO43-(aq) + 3Zn2+(aq) + 6NO3-(aq) ( 6NH4+(aq) + 6NO3-(aq) + Zn3(PO4)2(s) NIE: 2PO43-(aq) + 3Zn2+(aq) ( Zn3(PO4)2(s) 6.
Assume all reactions occur in aqueous solution. WebWrite the balanced net ionic equation for the reaction that takes place when aqueous solutions of the following solutes are mixed. If you add sodium carbonate solution to a solution of hexaaquairon(III) ions, you get exactly the same precipitate as if you added sodium hydroxide solution or ammonia solution. a. potassium chloride + mercury (I) acetate b. chromium (II) bromide + sodium carbonate c. copper (II) Nitrate + Magnesiu. A Identify the ions present in solution and write the products of each possible exchange reaction.
 Thus no net reaction will occur. Co(NO_3)_2 (aq) + NaCl (aq), Write the ionic and net ionic equations for the following reactions: a) AgNO3 + Na2SO4 b) BaCl2 + ZnSO4 c) (NH4)2CO3 + CaCl2 d) NaNO3 + KCl. First, we balance the molecular equation. For example: The reaction of potassium chloride and lead II nitrate
Molecular Equation: 2KCl (aq) + Pb(NO3)2 (aq) -> 2KNO3 (aq) + PbCl2 (s)
Complete Ionic Equation: 2K+ (aq) + 2Cl- (aq) + Pb2+ (aq) + 2NO3 (aq) -> 2K+ (aq) + 2NO3 (aq) + PbCl2 (s)
Net Ionic Equation: 2Cl- (aq) + Pb2+ (aq) -> PbCl2 (s)
Directions: Write balanced molecular, ionic, and net ionic equations for each of the following reactions. Get access to this video and our entire Q&A library, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations. Aqueous solutions of barium chloride and lithium sulfate are mixed.
Thus no net reaction will occur. Co(NO_3)_2 (aq) + NaCl (aq), Write the ionic and net ionic equations for the following reactions: a) AgNO3 + Na2SO4 b) BaCl2 + ZnSO4 c) (NH4)2CO3 + CaCl2 d) NaNO3 + KCl. First, we balance the molecular equation. For example: The reaction of potassium chloride and lead II nitrate
Molecular Equation: 2KCl (aq) + Pb(NO3)2 (aq) -> 2KNO3 (aq) + PbCl2 (s)
Complete Ionic Equation: 2K+ (aq) + 2Cl- (aq) + Pb2+ (aq) + 2NO3 (aq) -> 2K+ (aq) + 2NO3 (aq) + PbCl2 (s)
Net Ionic Equation: 2Cl- (aq) + Pb2+ (aq) -> PbCl2 (s)
Directions: Write balanced molecular, ionic, and net ionic equations for each of the following reactions. Get access to this video and our entire Q&A library, Precipitation Reactions: Predicting Precipitates and Net Ionic Equations. Aqueous solutions of barium chloride and lithium sulfate are mixed. When molecular compounds, such as sugar, dissolve in water, the individual molecules drift apart from each other. Ba (OH) 2 is also soluble. Aqueous solutions of rubidium hydroxide and cobalt(II) chloride are mixed. Write the complete ionic equation and net ionic equation for the reaction that occurs between sodium carbonate and cobalt(III) chloride. Simply mixing solutions of two different chemical substances does not guarantee that a reaction will take place.
@a?%%@b;ukFu|LU,y\yH*gf}~}qR$^-s-RESF~:;>g%gG H + (aq) + OH-(aq) H 2 O(l) Displacement When NaCl dissolves in water, the ions separate and go their own way in solution; the ions are now written with their respective charges, and the (aq) phase label emphasizes that they are dissolved (Figure \(\PageIndex{1}\)).
 Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. insoluble product that forms in a precipitation reaction. WebThere are three main steps for writing the net ionic equation for FeCl2 + Na2CO3 = FeCO3 + NaCl (Iron (II) chloride + Sodium carbonate). ^GO^}+i,T`I@u_ 6#q li|. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. The dissolving equation is Na. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between aqueous solutions of cobalt(II) sulfate and sodium carbonate. They are present, but they do not participate in the overall chemistry.
Molecular Equation: Complete Ionic Equation: Net Ionic Equation: Particulate drawing: Iron III chloride and magnesium metal. insoluble product that forms in a precipitation reaction. WebThere are three main steps for writing the net ionic equation for FeCl2 + Na2CO3 = FeCO3 + NaCl (Iron (II) chloride + Sodium carbonate). ^GO^}+i,T`I@u_ 6#q li|. In a precipitation reaction, a subclass of exchange reactions, an insoluble material (a precipitate) forms when solutions of two substances are mixed. The dissolving equation is Na. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction that occurs between aqueous solutions of cobalt(II) sulfate and sodium carbonate. They are present, but they do not participate in the overall chemistry. Ba (OH) 2 is also soluble. Learn to write ionic equations by following a molecular reaction. Web-- EXCEPT those also containing: sodium, potassium, ammonium* , or lithium (Na +, K +, NH 4 + or Li +) which are soluble. which property does hydrogen have that it is used in filling balloons?, In 5.8 moles of sucrose (C12H22O11) sample, If you add sodium carbonate solution to a solution of hexaaquairon(III) ions, you get exactly the same precipitate as if you added sodium hydroxide solution or ammonia solution. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
What was the concentration of each ion in the original solutions? Write the balanced molecular, ionic, and net ionic equations and specify reaction type. A When aqueous solutions of strontium bromide and aluminum nitrate are mixed, we initially obtain a solution that contains Sr2+, Br, Al3+, and NO3 ions. The ionic equation represents the reaction in terms of the dissociated ions, which helps to identify any precipitates or insoluble products that may form during the reaction. What mass of NaCl must be added to the 1500 L of silver waste to ensure that all the Ag+ ions precipitate? Later, this work was cited when Arrhenius was awarded the Nobel Prize in Chemistry.
Write the net ionic equation for the reaction involved in the testing of the anion. Molecular Equation: 2 KF(aq) + Mg(NO 3) 2 (aq) 2 KNO 3 (aq) + MgF 2 (s) Ionic Equation: 2 K+(aq) + 2 F-(aq) + Mg2+(aq) + 2NO 3-(aq) 2 K+(aq) + 2 NO 3-(aq) + MgF 2 (s) NIE: 2 F-(aq) + Mg2+(aq) MgF 2 (s) 16. Staff Login For example, if 500 mL of a 1.0 M aqueous NaCl solution is mixed with 500 mL of a 1.0 M aqueous KBr solution, the final solution has a volume of 1.00 L and contains 0.50 M Na+(aq), 0.50 M Cl(aq), 0.50 M K+(aq), and 0.50 M Br(aq). the Ag+(aq) and Cl(aq) ions become AgCl(s), but the Na+(aq) ions and the NO3(aq) ions stay as Na+(aq) ions and NO3(aq) ions. Given 1.24 liters of a 2.00 M solution of iron(II) chloride and unlimited sodium carbonate, how many grams of iron(II) carbonate can the reaction produce? A Because barium chloride and lithium sulfate are strong electrolytes, each dissociates completely in water to give a solution that contains the constituent anions and cations. The net ionic equation is Ni2+ + 2OH- Ni(OH)2.
 Write the net ionic equation for the reaction between aqueous solutions of calcium chloride and sodium carbonate. Which representation in Problem 3 best corresponds to an aqueous solution originally containing each of the following?
Write the net ionic equation for the reaction between aqueous solutions of calcium chloride and sodium carbonate. Which representation in Problem 3 best corresponds to an aqueous solution originally containing each of the following? Provide the molecular, ionic, and net ionic equations of the following: Nickel (II) chloride + Copper (II) sulfate. WebSodium carbonate and hydrochloric acid produces sodium chloride, carbon dioxide and water. Ionic compounds that dissolve separate into individual ions. Write the molecular equation, the ionic equation, and the net ionic equation for the reaction between barium chloride and sodium phosphate.
What is the percentage by mass of NaAsO2 in the original sample?
Provide the molecular equation and the net ionic equation for silver nitrate and sodium carbonate. Staff Login We know that 500 mL of solution produced 3.73 g of AgCl. WebThe chloride (Cl-) and potassium (K +) ions are spectator ions. This type of reaction is called a precipitation reaction, and the solid produced in the reaction is known as the precipitate. Web1. oli Q (yqTT Write molecular and net ionic equations for the following reaction. WebIn comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us about the ionic compounds that were used as the sources of \text {Ag}^+ Ag+ and \text {Cl}^- Cl for the reaction. Interestingly, his PhD examination team had a hard time believing that ionic compounds would behave like this, so they gave Arrhenius a barely passing grade. If no reaction occurs, so indicate. Thus Pb(C2H3O2)2 will dissolve, and PbI2 will precipitate. Legal. Write the balanced molecular equation, complete ionic equation, and net ionic equation for the reaction between aqueous solutions of sodium carbonate and barium chloride. The wing reaction produces this molecule.Cl2(g) + H2O(l) + HOCI(aq) + A) HOCl(aq) is the molecule that kills bacteria when chlorine is added to water. You dissolve a 10.00 g sample in water, oxidize it to arsenate, and dilute it with water to a final volume of 500 mL.
Obtain the mass of NaCl by multiplying the number of moles of NaCl needed by its molar mass. Predicting the solubility of ionic compounds in water can give insight into whether or not a reaction will occur. Provide the molecular, ionic, and net ionic equations of the following: Sodium nitrate + Sulfuric acid.
They do not remain as Cl2 (that would be elemental chlorine; these are chloride ions), and they do not stick together to make Cl2 or Cl22. WebSodium carbonate and Iron II chloride Chemical Equation: Complete Ionic Equation: Net Ionic Equation: This problem has been solved! Silver bromide is an off-white solid that turns black when exposed to light, which is due to the formation of small particles of silver metal.
Write the net ionic equation (only) that occurs when aqueous sodium carbonate solution is added to aqueous calcium chloride solution. For our purposes, however, we will assume that precipitation of an insoluble salt is complete. If no reaction is likely, explain why no reaction would be expected for that combination of solutes. Table 12.4.1 shows that LiCl is soluble in water (rules 1 and 4), but BaSO4 is not soluble in water (rule 5). For most ionic compounds, there is also a limit to the amount of compound that can be dissolved in a sample of water.
This behavior was first suggested by the Swedish chemist Svante August Arrhenius [18591927] as part of his PhD dissertation in 1884. Sodium carbonate and ammonium chloride is a combination of two ionic compounds that produces a double replacement reaction.
(Water molecules are omitted from molecular views of the solutions for clarity.). Write the balanced net ionic equation for the reaction that takes place when aqueous solutions of sodium carbonate and iron(III) chloride are mixed. WebThe balanced equation is NiCl2 + 2KOH Ni(OH)2 + 2KCl. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out.
The only possible exchange reaction is to form LiCl and BaSO4: B We now need to decide whether either of these products is insoluble. Write the balanced molecular equation, total ionic equation, and net ionic equation for the reaction that occurs when magnesium chloride and sodium carbonate are mixed. The resulting precipitate of Ag3AsO4 has a mass of 3.24 g after drying. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Net ionic equations show only the ions and other substances that change in a chemical reaction. Given 1.24 liters of a 2.00 M solution of iron(II) chloride and unlimited sodium carbonate, how many grams of iron(II) carbonate can the reaction produce? If no reaction occurs, complete the molecular and ionic equations, but write "no reaction" in place of the net ionic equation. WebSodium carbonate and Iron II chloride Molecular Equation: Na2CO3(aq) + FeCl2(aq) ( FeCO3(s) + 2NaCl(aq) Complete Ionic Equation: 2Na+(aq) + CO32-(aq) + Fe2+(aq) + 2Cl-(aq) ( FeCO3(s) Particulate drawing: Net Ionic Equation:CO32-(aq) + Fe2+(aq) ( FeCO3(s) Magnesium hydroxide and hydrochloric acid Molecular Equation: We will discuss solubilities quantitatively later on, where you will learn that very small amounts of the constituent ions remain in solution even after precipitation of an insoluble salt. Another place where solubility versus insolubility is an issue is the Grand Canyon.
u This is more representative of what is occurring in the solution. Because both components of each compound change partners, such reactions are sometimes called double-displacement reactions. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: sodium carbonate and copper (I) chloride. Polyatomic ions also retain their overall identity when they are dissolved. Because the solution also contains NH4+ and I ions, the possible products of an exchange reaction are ammonium acetate and lead(II) iodide: B According to Table 12.4.1 ammonium acetate is soluble (rules 1 and 3), but PbI2 is insoluble (rule 4). If you add sodium carbonate solution to a solution of hexaaquairon(III) ions, you get exactly the same precipitate as if you added sodium hydroxide solution or ammonia solution. If so, identify the precipitate. 3 0 obj
t: 6.0210^23 atoms] and so on.
Thus 78.1 mol of NaCl are needed to precipitate the silver. Give the molecular, ionic, and net ionic equations for potassium carbonate and hydroiodic acid. Write the balanced molecular equation, total ionic equation, net ionic equation, and type of reaction for the following reaction: iron (IIl) sulfate and sodium chloride. Prince George's Community College By removing the spectator ions, we are left with the net ionic equation. Solid lead(II) acetate is added to an aqueous solution of ammonium iodide. b. Aqueous sodium hydroxide and aqueous iron (III) chloride. endobj Solubility rules are very useful in determining which ionic compounds are dissolved and which are not.
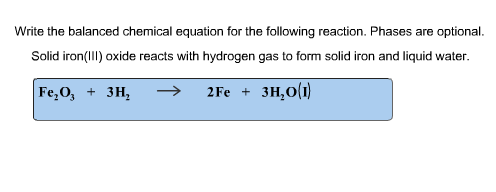 Provide the molecular, ionic, and net ionic equations of the following: Sodium carbonate + Hydrochloric acid.
Provide the molecular, ionic, and net ionic equations of the following: Sodium carbonate + Hydrochloric acid. Balance the equation and determine how many moles of O2 are required to react completely with 7.2 moles of C6H14. Underline all solids. It is common to cancel spectator ions (something also done with algebraic quantities) on the opposite sides of a chemical equation: \[\cancel{Na^{+}(aq)}+Cl^{-}(aq)+Ag^{+}(aq)+\cancel{NO_{3}^{-}}(aq)\rightarrow AgCl(s)+\cancel{Na}^{+}(aq)+\cancel{NO}_{3}^{-}(aq)\nonumber \]. and so on) Group of answer choices a) 1,3,3,2 b) 2,3,2,3 c) 1,3,2,3 d) 1,2,2,2 Find the molecular and ionic equations for __Iron (III) chloride + sodium nitrate__. WebWrite the ionic equation and net ionic equation for the following.
I just took the Plato Test, This site is using cookies under cookie policy . Write the molecular, ionic, and net ionic equations for the combination of Sr(NO3)2(aq) and Li2SO4(aq).