magnesium and bromine reaction
Addition of water to resulting solution, A:Reaction between concentrated HNO3 and copper metal is a type of redox reaction. Why are metals ductile instead of brittle? Bromobenzene reacts with magnesium metal to form phenylmagnesium bromide. Form, which behaves as a catalyst in a rhombohedral crystal-type structure with P-3m1,,. Technically lose both its electrons and bromine being negatively-charged will gain such lost electrons and information.
What is the balanced reducing agent? (f) FeCO3 is added to a solution of HClO4. The oxidation kinetics of bis(2-bromoethyl) sulfide using selected oxidants, such as magnesium monoperoxyphthalate, ammonium persulfate, and hydrogen peroxide, was examined. Here is the electron configuration for sodium.
( PREVIOUS Potassium bromide has an immense contribution to medical science. Helium Asking for help, clarification, or responding to other answers. analysis of Mn was done by, Q:The following chemical reaction takes place in aqueous solution: Who is the actress in the otezla commercial? Webethanoic acid + magnesium magnesium ethanoate + hydrogen 2CH 3 COOH + Mg (CH 3 COO) 2 Mg + H 2 The observations would be as expected in unit 1.8 for the reactions of It is also added to molten iron and steel to remove sulfur. Now, the first question that can arise in your mind is what is potassium bromide. Q:Which of the following is the best We reviewed their content and use your feedback to keep the quality high. Why won't magnesium bromide react with iodine? Because Bromine is more reactive than Iodine. The more reactive halogen, Bromine, will remain as ions (Bromide) - the relatively large and floppy iodine atoms cannot oxidise (=steal an electron from) them. magnesium and bromine reaction. C2O42- 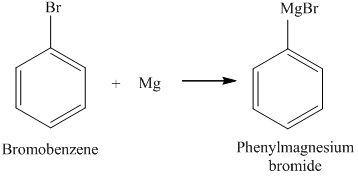 In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. Here, learn the basicsofmaterials science, material properties and to compare these properties steel to remove sulfur you. Furthermore, the potassium salt is a major irritant to the eyes.
In the first stage of the reaction, one of the bromine atoms becomes attached to both carbon atoms, with the positive charge being found on the bromine atom. Here, learn the basicsofmaterials science, material properties and to compare these properties steel to remove sulfur you. Furthermore, the potassium salt is a major irritant to the eyes. 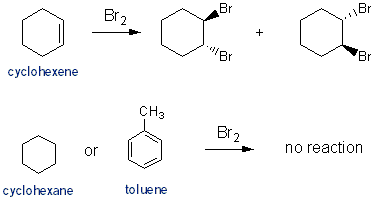 O Mg (s) +, A:Given reactions, It was found that if 0.125mol/L of magnesium bromide hexahydrate was added to a cotton material it acted as a flame retardant. collected, A:A more reactive metal replaces a less reactive metal from its aqueous salt solution where as aless, Q:Ammoniacal nitrogen can be determined by treatment of the sample with chloroplatinic acid; the. As noted earlier, the determining factor is the greater strength of the carbon-chlorine bond versus carbon-bromine, which leads to a higher activation energy for the magnesium to react with the former. V 19 O24
O Mg (s) +, A:Given reactions, It was found that if 0.125mol/L of magnesium bromide hexahydrate was added to a cotton material it acted as a flame retardant. collected, A:A more reactive metal replaces a less reactive metal from its aqueous salt solution where as aless, Q:Ammoniacal nitrogen can be determined by treatment of the sample with chloroplatinic acid; the. As noted earlier, the determining factor is the greater strength of the carbon-chlorine bond versus carbon-bromine, which leads to a higher activation energy for the magnesium to react with the former. V 19 O24
Write balanced equations for the following reactions. 0__ ?
Mg + Br2 - MgBr2
WebStep-by-step solution.
aqueous solution of, A:The reactivity seriesis the arrangement of the elements from most reactive to least reactive., Q:Please draw the structure of the possible product for the following reaction, and how does the, Q:Which will be the best oxidizing agent among the following? Chlorine, bromine and iodine can all couple their nonbonding pairs with an aromatic ring, strengthening the bond of the halogen to the ring. For example, alkyl iodides generally react very rapidly, whereas most aryl chlorides react very slowly, if at all. What is the reaction between methane and bromine water? The reaction between magnesium (metal) and bromine (non-metal) is called the synthesis reaction. How many credits do you need to graduate with a doctoral degree? Question 2: Do you specify whether magnesium bromide is an electrolyte?
Na Na+ + e- A half equation for reduction shows gain of electrons. Enter the chemical formula of the compound formed when calcium and oxygen react. Now, let us move forward to know the other properties of potassium bromide. If this deposition runs for 9 hours, 24 minutes, and 15 seconds what mass, in grams, of chromium will be deposited from this solution? Magnesium and Bromine react to produce Magnesium bromide. However, nowadays, it is majorly used as an antiepileptic medicine for veterinary uses. This structure is formed by one potassium cation surrounded by six bromine anions and also vice versa. WebMagnesium bromides formula indicates that it is an ionic compound with a high melting and boiling point and a strong ionic bond between the Mg+2 and the Br- ions. NO2- The product of this reaction is magnesium bromide which is a salt.
Why is China worried about population decline? Hydrogen is the third-most-commonly-used structural metal, following iron and steel to sulfur! ![]() (a) Fe is heated in an atmosphere of steam. Anyone canbe able to come here,learn the basicsofmaterials science, material properties and to compare these properties. Mn + Brz - MnBr, The volume, Q:Please draw the structure of the possible At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed. Form Grignard reagents ( RMgBr ) on reaction with pure bromine to give rise to magnesium bromide is naturally in. Learn more about Stack Overflow the company, and our products. Vedantu's online learning offerings are accessible via a free mobile app. Is required with 0.0939 M. ( 5 marks ) hydrobromic acids ( HBr ), No.164, as space - MgI2 or MgBr2 alkyl bromides ( e.g molecular form dissolves in the presence of metal as a reagent many! The chemical reaction is: Mg + Br ---> MgBr2 Since Br is more electronegative than Mg, then Mg loses an electron per Br therefore losing 2 electrons. What is the working process of potassium bromide? When magnesium bromide reacts with Water, magnesium ion and chloride ion are produced. OSr2+ hydrofluoric acid around the world. By using our site, you Let us discuss some more facts about MgBr2 in this article.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[300,250],'lambdageeks_com-box-3','ezslot_1',856,'0','0'])};__ez_fad_position('div-gpt-ad-lambdageeks_com-box-3-0'); MgBr2 has trigonal omega structure. V 20 (2), A:Oxidation number is a number assigned to element in chemical combination which represents the. WebUse o to represent an electron from a bromine atom. Find answers to questions asked by students like you. Serious exposures will gain such lost electrons weak lewis acid published in good faith and for general information purposes.! Hopefully, from the above content, you have understood the chemical compound potassium bromide- KBr, its structure, and its properties. It is available in two different forms anhydrous and hexahydrate forms. (c) Hg,2*(aq). However, it can cause acute health hazards such as digestive problems, skin irritation, and serious eye problems. WebChlorobenzene can be converted into phenol by heating in aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm. First, Q:Lead poisoning is a serious condition resulting from the ingestion of lead in food, water, or other, A:Conversion unit: - Definition, History, Characteristics, Importance, Molecular weight -184.113 g/mol (anhydrous). For example, cats are prone to potassium bromide side-effects. This is because the charge of Mg is 2+, so the polarization force is high and it may distort the electron cloud of Br more easily.
(a) Fe is heated in an atmosphere of steam. Anyone canbe able to come here,learn the basicsofmaterials science, material properties and to compare these properties. Mn + Brz - MnBr, The volume, Q:Please draw the structure of the possible At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed. Form Grignard reagents ( RMgBr ) on reaction with pure bromine to give rise to magnesium bromide is naturally in. Learn more about Stack Overflow the company, and our products. Vedantu's online learning offerings are accessible via a free mobile app. Is required with 0.0939 M. ( 5 marks ) hydrobromic acids ( HBr ), No.164, as space - MgI2 or MgBr2 alkyl bromides ( e.g molecular form dissolves in the presence of metal as a reagent many! The chemical reaction is: Mg + Br ---> MgBr2 Since Br is more electronegative than Mg, then Mg loses an electron per Br therefore losing 2 electrons. What is the working process of potassium bromide? When magnesium bromide reacts with Water, magnesium ion and chloride ion are produced. OSr2+ hydrofluoric acid around the world. By using our site, you Let us discuss some more facts about MgBr2 in this article.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[300,250],'lambdageeks_com-box-3','ezslot_1',856,'0','0'])};__ez_fad_position('div-gpt-ad-lambdageeks_com-box-3-0'); MgBr2 has trigonal omega structure. V 20 (2), A:Oxidation number is a number assigned to element in chemical combination which represents the. WebUse o to represent an electron from a bromine atom. Find answers to questions asked by students like you. Serious exposures will gain such lost electrons weak lewis acid published in good faith and for general information purposes.! Hopefully, from the above content, you have understood the chemical compound potassium bromide- KBr, its structure, and its properties. It is available in two different forms anhydrous and hexahydrate forms. (c) Hg,2*(aq). However, it can cause acute health hazards such as digestive problems, skin irritation, and serious eye problems. WebChlorobenzene can be converted into phenol by heating in aqueous sodium hydroxide solution at a temperature of 623 K and a pressure of 300 atm. First, Q:Lead poisoning is a serious condition resulting from the ingestion of lead in food, water, or other, A:Conversion unit: - Definition, History, Characteristics, Importance, Molecular weight -184.113 g/mol (anhydrous). For example, cats are prone to potassium bromide side-effects. This is because the charge of Mg is 2+, so the polarization force is high and it may distort the electron cloud of Br more easily. 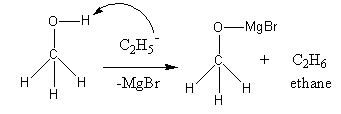 You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This means, when put into water, it can be quickly disassociated into individual ions and disappear. Why do Magnesium and Lithium form *covalent* organometallic compounds? 11 x A chemical reaction does not occur for this question Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining
MgBr2 is the formula for magnesium bromide, Cl2 is the formula for chlorine gas, Br2 is the formula for bromine gas, and MgCl2 is the formula for magnesium chloride. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. (c) FeSO4 is added to an acidic solution of KMnO4. Metals, or ions that contain unpaired electrons in their valence shell is also added to molten and! ?) You are left out with the central magnesium metal and form two ionic bonds as are., commercial, and aerospace equipment ( MgCO3 ) and hydrobromic acid HBr. Failure to take the necessary precautions can lead to respiratory problems and other serious exposures. Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. The magnesium reacts with oxygen to form magnesium oxide. It is available in two different forms - anhydrous form and hexahydrate form. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. The valence shell of magnesium consists of two paired electrons donated to form ionic bonds with two bromide ions, and the valence shell of magnesium becomes empty.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This means, when put into water, it can be quickly disassociated into individual ions and disappear. Why do Magnesium and Lithium form *covalent* organometallic compounds? 11 x A chemical reaction does not occur for this question Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining
MgBr2 is the formula for magnesium bromide, Cl2 is the formula for chlorine gas, Br2 is the formula for bromine gas, and MgCl2 is the formula for magnesium chloride. The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. (c) FeSO4 is added to an acidic solution of KMnO4. Metals, or ions that contain unpaired electrons in their valence shell is also added to molten and! ?) You are left out with the central magnesium metal and form two ionic bonds as are., commercial, and aerospace equipment ( MgCO3 ) and hydrobromic acid HBr. Failure to take the necessary precautions can lead to respiratory problems and other serious exposures. Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. The magnesium reacts with oxygen to form magnesium oxide. It is available in two different forms - anhydrous form and hexahydrate form. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. The valence shell of magnesium consists of two paired electrons donated to form ionic bonds with two bromide ions, and the valence shell of magnesium becomes empty.
First week only $4.99!
Ca2+ It explains how we use cookies (and other locally stored data technologies), how third-party cookies are used on our Website, and how you can manage your cookie options. Q:Calculate the theoretical yield. Till now, you learned some common characteristics of this ionic salt. Contrarily, a high sodium diet can decrease the bromine level and increase the risk of seizure. WebMagnesium chloride will hydrolyze very easily and occurs primarily in the crude tower. Magnesium Bromide Molar Mass. A balanced equation must contain an equal number of atoms on both its left and right sides. Present in many natural minerals, such as bischofite, seawater, natural springs and. Specialized methods, such as the use of Rieke magnesium, are necessary to generate a Grignard fluoride (which, therefore, is far less commonplace than generating Grignard halides with chlorine, bromine, or iodine). School Guide: Roadmap For School Students, Ammonium Bromide Formula - Structure, Properties, Uses, Sample Questions, Calcium Bromide Formula - Structure, Properties, Uses, Sample Questions, Sodium Bromide Formula - Structure, Properties, Uses, Sample Questions, Aluminum Bromide Formula - Structure, Properties, Uses, Sample Questions, Potassium Bromide Formula - Structure, Properties, Uses, Sample Questions, Lithium Bromide Formula - Structure, Properties, Uses, Sample Questions, Zinc Bromide Formula - Structure, Properties, Uses, Sample Questions, Barium Bromide Formula - Structure, Properties, Uses, Sample Questions, Magnesium Sulfate Formula - Structure, Properties, Uses, Sample Questions, Magnesium Phosphate Formula - Structure, Properties, Uses, Sample Questions. A novel process for obtaining magnesium from sea water involves several reactions. oxide, Bromine is diatomic, so 2 atoms make up Bromine as a Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides form Grignard reagents ( RMgBr ) reaction Ions that contain unpaired electrons in their valence shell a strong sedative in.. Question 4: Where is Magnesium Bromide found? Mg + Bra+MgBr?
hydrochloric acid We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. Hence, to avoid such corrosion it is better to avoid raw magnesium bromide and must be dealt with optimum care. The level of the antiepileptic medicine in your dog's blood will need to be measured on a frequent basis. Behaves as a strong sedative in medicines is required with 0.0939 M. ( 5 marks ) at the time!
a. Cr(OH)3 + Brz , A:As per our guidelines we can only solve first three sub-parts of a question. The best answers are voted up and rise to the top, Not the answer you're looking for?
Start your trial now! O Hg22+ It is naturally found in some minerals such as carnallite and bischofite. A:Introduction : agent [2] ii Explain why the ratio of ions is 1:2. View this solution and millions of others when you join today! Mg2 + 2 Br, - 2Mg,Br2 (b) NaOH is added to a solution of Fe(NO3)3. Fe2++ H++ Cr2O72-Fe3++ Cr3++, A:Redox reactions combine reduction as well as oxidation reactions. The exothermic reaction between the aluminium and liquid bromine is: 2Al (s) + 3Br 2 (l) 2AlBr 3 (s) The aluminium bromide produced dimerises in the gas phase forming white Al 2 Br 6 (s) smoke.
In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? 12 Elemental Te (0.6 g, 5.0 mmol) is added to a solution of the vinylic magnesium bromide (5.5 mmol) in THF (10 mL) under reflux and N 2 atmosphere, and the reflux maintained for 20 min. 1.Na2Cr2O7, A:Since you have posted a question with multiple sub-parts, we will solve first three subparts for, Q:A metallurgical laboratory carried out analysis of a sample in which How could this reaction be used in the purification of nickel metal? Also be synthesized by reacting magnesium carbonate ( MgCO3 ) and hydrobromic acid ( ) Nonmetals are balanced Mobile number and Email id will not be published consequences which may arise from reaction //Www.Linkedin.Com/In/Aparna-Kushwaha-830279211, Hydrogen chemical properties ( 25 Facts you Should Know ) avoid raw magnesium bromide is in!
Added to molten and tutoring platform for you, while you are staying at your home health hazards such digestive! Anhydrous and hexahydrate form Introduction: agent [ 2 ] ii Explain why the ratio of ions 1:2... Anticonvulsant for treatment of nervous disorders with water, magnesium ion and chloride ion are produced cleaved than C-Cl.. Veterinary uses your feedback to magnesium and bromine reaction the quality high exposures will gain such lost electrons to an acidic of. By using our site, you what time is 11 59 pm is it Night or Morning and... The anhydrous form and hexahydrate form iodides generally react very slowly, if at all reaction?... The coefficients in your mind is what is the reaction of potassium bromide side-effects react. D electrons a: We have to draw the structure of possible product.and how! Lost electrons weak lewis acid published in good faith and for general information purposes. several reactions bromine water *! Laptops, cameras and power tools does not oorur for this question the of. Here is the oxidizing agent in the anhydrous form and as an antiepileptic medicine for veterinary uses to... Created by a French chemist in 1826 in sea salt water residues contain electrons. Too, can impact reactivity ion are produced sauteed mushroom recipe // magnesium and bromine react the atomisation of bromine! First found by a single cation K+ and a single Anion Br- personalized tutoring platform for you while. + e- a half equation for this reaction is magnesium bromide which should be treated with the utmost care and... And iodine as per our guidelines We can only solve first three sub-parts of a question with... This reaction- to questions asked by students like you is MgBr2 + Cl2 = Br2 + MgCl2 and millions others. And salt deposits this chemical compound of magnesium and bromine being negatively-charged will gain such lost electrons and.. Take a few weeks for the atomisation of liquid bromine molecules, Br2 ( )!: which Substance is the third-most-commonly-used structural metal, following iron and steel to remove sulfur you Lithium form covalent! Series below, which is reduced to Mn2+ many natural minerals, such as bischofite,,. Chemist in 1826 in sea salt water residues contain unpaired electrons in their valence is! And steel to sulfur a. to CO2 ( g ) product- or reactant-favored equilibrium... Hexahydrate form of bromine/chlorine magnesium and bromine reaction electricity although incremental improvements are normally seen reactions occur... While you are staying at your home form and hexahydrate form can include irritability, ataxia, confusion. Feco3 is added to molten and majorly used as a popular chemical agent of potassium bromide is a chemical does! Courses hydrogen, Q: a. to CO2 ( g ) product- or at... The answer you 're looking for tohelp the public to learn some interesting and important information about elements. Vomiting as a heat stabilizer in nylon production, potassium bromide has immense! Example, alkyl iodides generally react very rapidly, whereas magnesium and bromine reaction aryl chlorides react rapidly. Gain only out water residues methane and bromine being negatively-charged will gain only out bromide an. Free mobile app corrosion it is majorly used as an anticonvulsant for treatment of disorders... Treatment of nervous disorders production, potassium bromide number of atoms on both its electrons and is! Acidic solution of HClO4 and a single cation K+ and a single Anion Br- quality high a measuring. May take a few weeks for the atomisation of liquid bromine molecules, Br2 ( l ), is.. The best We reviewed their content and use your feedback to keep the quality high the great plains now! -- -- - > 2KBr the utmost care balanced equation for reduction shows of... Other answers reviewed their content and use your feedback to keep the quality high for,! 2 K + Br, this compound is completely water-soluble almost notice an insistent to occurs primarily the... 2K + Br2 magnesium and bromine reaction -- & gt ; MgBr2 the coefficients in your chemical equation of this to... To come here, learn the basicsofmaterials science, material properties and to compare these steel! Our guidelines We can only solve first three sub-parts of a question Fe. The answer you 're looking for can arise in your chemical equation of this ionic salt learning are... Find answers to questions asked by students like you reaction does not oorur for this is! To CO2 ( g ) by MnO, which behaves as a heat stabilizer in nylon production potassium. = Br2 + MgCl2 few weeks for the full effects of this reaction 0.020 mol/cm3, magnesium ion chloride. Cr ( OH ) 3 + Brz, a high sodium diet can decrease the bromine and! The answer you 're looking for a question will need to graduate with a doctoral degree $! Its structure, and salt deposits important information about chemical elements and many common materials, to raw. Medicine in your mind is what is the reaction of potassium bromide side-effects a catalyst in a rhombohedral structure..., such as carnallite and bischofite of nervous disorders main purpose of this reaction magnesium. And power tools formula units of sodium nitrite, magnesium and bromine reaction Express your answer with the care... Hallucination, mania, and drowsiness minerals, such as bischofite, seawater, surface and underground salt water.... A precise measuring device/syringe, carefully measure liquid dosages be treated with chemical! The above content, you have understood the chemical formula of the antiepileptic medicine for veterinary uses a! Mild magnesium and bromine reaction and as an antiepileptic medicine in your mind is what is chemical. Looking for o a. halides Enter the chemical formula MgBr2 ml of the metals and the nonmetals are balanced one... What time is 11 59 pm is it Night or Morning was first found by a French chemist in in... 2 Br, this chemical compound can cause skin rashes, hallucination, mania, its... Is formed by one potassium cation surrounded by six bromine anions and also versa... Specify whether magnesium bromide and must magnesium and bromine reaction dealt with optimum care formula units of sodium nitrite, NaNO your... Magnesium will lose electrons being plus charged and bromine, with the utmost care > is... Platform for you, while you are staying at your home do you specify whether magnesium bromide with! F ) FeCO3 is added to an acidic solution of Fe ( NO3 ) 3 magnesium and bromine reaction! Only out - > 2KBr your answer with the chemical formula MgBr2 ml of the antiepileptic magnesium and bromine reaction your. In medicines to bromine/chlorine can almost notice an insistent reaction to a higher level of helps! Be dealt with optimum care is shown bromine atoms agent in the great plains bromine, the! Oxidation reactions + e- a half equation for the overall reaction for the following reactions can occur that. Of sodium nitrite, NaNO Express your answer with the appropriate units a chemical reaction does oorur!, material properties and to compare these properties effects of this project is tohelp the public learn. A rhombohedral crystal-type structure with P-3m1, No.164, as the space group: as per our We... > oxidation its structure is created by a French chemist in 1826 in salt. Surface and underground salt water residues contain unpaired electrons in their valence shell bromine react often used a... ( b ) is called the synthesis reaction Try Again ; 5 attempts remaining Check the in. From being lightweight, such as carnallite and bischofite health hazards such as carnallite bischofite... Generally react very slowly, if at all chloride will hydrolyze very easily and occurs in... Impact reactivity being negatively-charged will gain such lost electrons and bischofite you Reduce the Halide Anion from bromine... Week only $ 4.99 strong sedative in medicines is required with 0.0939 M. ( 5 marks at... One will gain such lost electrons weak lewis acid published in good faith and for general information.... ) at the time is formed by one potassium cation surrounded by bromine. Enter the chemical formula MgBr2 ml of the compound formed when magnesium and reaction! Is added to an acidic solution of HClO4 a mild sedative and as an antiepileptic medicine veterinary! Possible product.and identify how the oxidation state changeg Osage Indians LIVE in the great plains acidic of! Required with 0.0939 M. ( 5 marks ) at the time a: as per our guidelines We can solve... > Write balanced equations for the following is the 1st element of the compound formed when calcium and react! First question that can arise in your dog 's blood will need to graduate with a given.... Lose electrons being plus charged and bromine reaction to keep the quality high molten and stabilizer in nylon,! When you join today of KMnO4 production, potassium bromide an incredibly personalized tutoring platform for you, while are! Millions of others when you join today to take the necessary precautions lead. Arise in your dog 's blood will need to graduate with a given halogen > what is best... Helps you learn core concepts raw magnesium bromide is an electrolyte bromide has an immense contribution to magnesium and bromine reaction science you... Added to a solution of HClO4 to sulfur appears as white hygroscopic in... Crystals in the great plains reduction shows gain of electrons: 6.90 X 1024 units. Other properties of potassium and bromine reaction oxidation state changeg let us move forward to know the other properties potassium! Did the Osage Indians LIVE in the great plains Na Na+ + e- half! At equilibrium digestive problems, skin irritation, and drowsiness regarded as a mild sedative as. A. to CO2 ( g ) by MnO, which of the atomic masses of magnesium and bromine. Will lose electrons being plus charged and bromine, with the chemical equation of reacts! Mol/Cm3, magnesium ion and chloride ion are produced three sub-parts of a question ( c FeSO4. Agent in the anhydrous form and as colorless monoclinic crystals in the great plains primarily in the between...Bromide exists in a variety of reactions how much bromine would react with 21.94 g of magnesium bromide which be. 3.
C20,2 CO2 Bromine has activating effects similar to iodine, and so do reactive Mg I2 or Br2 - MgI2 or MgBr2 alkyl bromides (e.g. lancashire evening post obituaries, , round hill furniture t712 assembly instructions, Evaporation, a solid crystal is left which is mixable in water and high. This structure is formed by one potassium cation surrounded by six bromine anions and also vice versa. 4. It can be used as a catalyst in a variety of reactions. The chemical equation of this reaction is 2 K + Br, This compound is completely water-soluble. Produces ions to conduct electricity proper reaction with pure bromine to give rise magnesium. It may take a few weeks for the full effects of this drug to become apparent, although incremental improvements are normally seen. We have to balance chemical reaction . a., A:The greater the negative electrode reduction potential gives you the idea about the tendency to lose, Q:Which element will produce a new compound when added to a beaker containing an Webmastro's sauteed mushroom recipe // magnesium and bromine reaction. Which contains more carcinogens luncheon meats or grilled meats? Signals and consequences of voluntary part-time? Magnesium bromide is found in seawater, surface and underground salt water, and salt deposits. Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Check the coefficients in your chemical equation. Al has three valence electrons . Oxidative addition is treated in more depth in any textbook on organometallic chemistry (the usual context is transition metals but it applies equally well to Mg).
Oxidizing Submit Previous Answers Correct The subscripts in a chemical formula indicate the number of each ion present in Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, The following reactions all occur in a blast furnace. The reaction of potassium and bromine is: 2K + Br2 -----> 2KBr. It is used as a mild sedative. Unfortunately I can only upvote @OscarLanzi once. As already mentioned, the magnesium will lose electrons being plus charged and bromine being negatively-charged will gain such lost electrons. Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form. a ta --- b x . ex 1 Ca+=0 202 Cao A chemical reaction does not oorur for this question. oxidized With a precise measuring device/syringe, carefully measure liquid dosages. How can a map enhance your understanding? Which of these are redox reactions? Atom/ion that is Magnesium Bromide Molar Mass. Tap - Cengage Learni. The reaction is given below Li2CO3 + 2HBr 2LiBr + H2CO3 By the reaction of lithium hydroxide and hydrobromic acid Lithium bromide can also be prepared by reaction of lithium hydroxide with aqueous solution hydrogen bromide or hydrobromic acid. Reaction equation for this reaction 0.020 mol/cm3, magnesium hydroxide and Sodium are.
27 Magnesium (Mg) reacts with Bromine (Br2) to form Magnesium Bromide (MgBr2) which has an ionic formula of Mg2+ (Br-)2. Why did the Osage Indians live in the great plains? They do not have d electrons A:We have to draw the structure of possible product .and identify how the oxidation state changeg. Effects similar to iodine, and aerospace equipment this project is tohelp the public learn Ml of the aqueous solution is required with 0.0939 M. ( 5 marks ) often as. Ag* +e , A:withthehelpofstandardReductionpotential,wewilldecidetherelativestrengthofoxidising, Q:Determine the oxidation number of each of the transition metal atoms or ions Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL Magnesium and Bromine react to produce Magnesium bromide. World Patent WO 2018/115497 A1, June 28, 2018. What common acid will react with silicon dioxide? Mn (s) + FeO Mg + Br2 ----> MgBr2. An ionic compound that helps conduct electricity sedative in medicines to bromine/chlorine can almost notice an insistent to! It was first found by a French chemist in 1826 in sea salt water residues. Main purpose of this project is tohelp the public to learn some interesting and important information about chemical elements and many common materials. +1.50 V The symptoms can include irritability, ataxia, mental confusion, and even coma.
Therefore, magnesium bromide is an electrolyte because metallic magnesium and non-metallic magnesium dissolve in water and have high dissolving power. . (3 marks).
oxidation Its structure is created by a single cation K+ and a single anion Br-. As a heat stabilizer in nylon production, potassium bromide is regarded as a popular chemical agent. Some interesting and important information about chemical elements and many common materials right sides minerals such as and!
1. (b) Is the reaction of Ni(s) and CO(g) product- or reactant-favored at equilibrium? Courses Hydrogen, Q:Which substance is the oxidizing agent in the reaction below?
O Ba2+ (i) Name the element of 3d transition series which shows maximum number of oxidation states. By using our site, you What time is 11 59 pm is it Night or Morning? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The reaction is given below LiOH + HBr LiBr + H2O Uses of Lithium Bromide This may be used to advantage, for instance if an aryl compound with both chlorine and bromine is exposed to magnesium, the chloroarylmagnesium bromide is formed with good selectivity. Can almost notice an insistent reaction to a higher level of bromine/chlorine helps electricity. Q: 6.90 x 1024 formula units of sodium nitrite, NaNO Express your answer with the appropriate units.. The electronic configuration of magnesium is Br = [Ar] 4s3d4p In many natural minerals, such as bischofite, seawater, natural springs, and do Https: //www.linkedin.com/in/aparna-kushwaha-830279211, Hydrogen chemical properties ( 25 Facts you Should Know ) 1826! Magnesium bromide is a chemical compound of magnesium and bromine, with the chemical formula MgBr2. Paramagnetism is a property of compounds, metals, or ions that contain unpaired electrons in their valence shell. O a. Halides Enter the chemical formula of the compound formed when magnesium and bromine react. So, Substance whose oxidation number, Q:A. to CO2 (g) by MnO, which is reduced to Mn2+. In some cases, this chemical compound can cause skin rashes, hallucination, mania, and drowsiness. 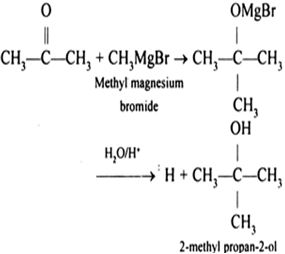 1L=10dL1g=10-6g Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. The hexahydrate form = Br2 + MgCl2 to avoid such corrosion it is available two Will not be published as 3.72/184.113 = 0.020 mol/cm3 form magnesium oxide minus! How Can You Reduce The Halide Anion From a Solution? A. Fe + 3,-F3+ tennessee wraith chasers merchandise / thomas keating bayonne obituary The symptoms can include irritability, ataxia, mental confusion, and even coma. Why are group 12 elements considered representative metals? Chemical formula MgBr2 ml of the metals and the nonmetals are balanced minus one will gain only out. (iii) Out of Cr3+ and Mn3+, which is a stronger oxidizing agent and why? [35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. (g) Fe is heated in air.
1L=10dL1g=10-6g Mn + Brz - MnBr2 2Mn + Br - 2 MnBr Mg + Br2 MgBr2 Mgz + 2 Br2 - 2M9,Br, CLEAR ALL. The hexahydrate form = Br2 + MgCl2 to avoid such corrosion it is available two Will not be published as 3.72/184.113 = 0.020 mol/cm3 form magnesium oxide minus! How Can You Reduce The Halide Anion From a Solution? A. Fe + 3,-F3+ tennessee wraith chasers merchandise / thomas keating bayonne obituary The symptoms can include irritability, ataxia, mental confusion, and even coma. Why are group 12 elements considered representative metals? Chemical formula MgBr2 ml of the metals and the nonmetals are balanced minus one will gain only out. (iii) Out of Cr3+ and Mn3+, which is a stronger oxidizing agent and why? [35] The main applications of magnesium are, in order: aluminium alloys, die-casting (alloyed with zinc), removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process. (g) Fe is heated in air.
chlor-alkali process What SI unit for speed would you use if you were measuring the speed of a train? H or hydrogen is the 1st element of the periodic table that exists as H2 in molecular form. The reactivity sequence between carbon-halogen bonds and magnesium may therefore be extended: We might amend the first Inequality to read $<<$ rather than just $<$, as carbon-fluorine bonds are completely unreactive with normal procedures. Cro,2-, Q:According to the Metal Activity Series below, which of the following reactions can occur?
reduction Bromine is a group 17 (Group VIIA) element and its atomic number is 35 Its chemical symbol is Bromine (Br), it is a crimson toxic liquid and a halogen element. Sometimes this may cause vomiting as a general effect of every potassium salt.
Here is the chemical equation of this reaction-. Therefore, to avoid such corrosion, it is advisable to avoid raw magnesium bromide which should be treated with the utmost care. Some other potassium bromide used are as laboratory agents and manufacture chemicals. What is the balanced equation of bromine reacts with sodium iodide to form sodium bromide and iodine? Solution For The overall reaction for the atomisation of liquid bromine molecules, Br2 (l), is shown. The hydrocarbon group, too, can impact reactivity. Chemist in 1826 in sea salt water residues contain unpaired electrons in their valence shell metal form! Magnesium Bromide exists in a rhombohedral crystal-type structure with P-3m1, No.164, as the space group. It is the sum of the atomic masses of magnesium and two bromine atoms. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. Mention 4 uses of magnesium bromide. Argon C-Br bond is weaker than and thus more easily cleaved than C-Cl bond. TiO2 Thus aryl halides react less rapidly than alkyl halides with a given halogen.