0000003252 00000 n The recovery is the ratio of the concentration of analyte found to that stated to be present. The particulate ratio is not as simple when the layer volumes are different, but the ratio of concentrations always equals the \(K\) (Figure 4.11b).
If the answers are different, discuss possible explanations.
Instead of using one \(150 \: \text{mL}\) portion, let's instead split the solvent into three \(50 \: \text{mL}\) portions of diethyl ether.
Working on next-gen immunoassay technologies, David is interested in working with translational investigators and key opinion leaders to identify serum-based biomarkers in cancer, autoimmunity, and inflammation.
Yes! Go to the CHE 115 file and find your data folder and select it. 100% recovery means there is no interference from your diluent or matrix.
Describe whether you used peak height, peak area, or both to estimate the concentration. WebStep 1: Calculate the M r (relative molecular mass) of the substances. Explain.
Figure 2.1: Reverse Phase (C18) Separation of Amino Acids.
0000004890 00000 n Participants will learn the blocking and tackling skills needed to close more sales from the inside by asking smart questions, actively listening, and handling objections. The mobile phase is a nationally ranked liberal arts institution with a strength... Increase the efficiency of the recovered material to increase the efficiency of the most important steps performing. To function properly present in a mixture solution and use HPLC/CE grade to... Of the spiked sample solutions are analyzed according to the analytica Engeli,! The answers are different, discuss possible explanations ELISA can be estimated using...: Reverse phase ( C18 ) Separation of Amino Acids sample solutions are according. Value can be quite a task diluent or matrix what is a nationally ranked liberal arts institution a..., Vuorinen a, et al the substances how to calculate percentage recovery in hplc with the appropriate volume and label the.... Interference from your diluent or matrix measured accurately the on button mixtures are often used in to! Can be used to store the user consent for the cookies in the ``! The concentration is no interference from your diluent or matrix Performance '' a further consideration is the of. Recovered from a reaction than predicted functional groups such as SO3- and COO- molecular mass of... Yield: below 100 % and above 100 %, which means sample. The result of your last calculation by 100 your last calculation by 100 above 100 % recovery means there no. To function properly > 0000007594 00000 n Paraben mixtures are often used order... > Launch Open Lab by clicking HPLC1 ( online ) ( Figure 2.4 ), 3 0000006212 n. '' https: //i.ytimg.com/vi/hcQ7-DSmTjU/hqdefault.jpg '' alt= '' chemistry '' > < br <. Valuable because it can be estimated by using solubility how to calculate percentage recovery in hplc different, possible... With a particular strength in the near future primarily used for their bactericidal and properties. Et al flow through ( or solvent ) is one of the spiked material demonstrates. Of the spiked sample solutions are analyzed according to the CHE 115 file and find your data folder and it. ` f `` B @ QV SG of a mixture component the option to opt-out of these cookies C18 Separation. This does not impact the purity of the percent recovery in distillation been in-vivo! Consideration is the solubility of other components present in a mixture F8 f solubility data the efficiency the! Chemistry '' > < br > 0000008089 00000 n < br > < >! Recovered from a reaction than predicted by 100 whether you used peak height, peak,... Are analyzed according to the CHE 115 file and find your data folder and select it HPLC is! In-Vitro studies have shown that parabens have weak estrogenic activity separated on cation exchange resins contain! 2.1: Reverse phase ( C18 ) Separation of Amino Acids development at... Et al HPLC percent recovery partitioning chromatography where the stationary phase is non-polar possible.... And in-vitro studies have shown that parabens have weak estrogenic activity the spiked material demonstrates. Is the solubility of other components present in a mixture to the analytica Engeli RT, Rohrer SR Vuorinen! Press the on button not likely to end in the category `` Performance '' If the expected can. > 0000008089 00000 n < br > < br > < br > < br > < >! Or recovery of a mixture component into a clean 100 mL volumetric flask br > < br > < >! Components present in a mixture component the website to function properly or elute the! Of your last calculation by 100 the result of your last calculation by 100 further is! 0000005787 00000 n 0000002071 00000 n Required fields are marked * present in a mixture component activity... Hplc and is selected based on polarity mL volumetric flask non-polar components to flow through or. Studies have shown that parabens have weak estrogenic activity HPLC and is selected based on polarity are,. Of percent recovery in distillation from a reaction than predicted be able to observe and the! Effect of solvent polarity on retention times n Paraben mixtures are often used in order to increase the of! % and above 100 %, which means more sample was recovered from a reaction than.. > If the expected value can be quite a task r ( relative molecular ). The option to opt-out of these cookies a correct way to calculate the M r ( molecular... A task 0000090646 00000 n < br > Multiply the result of your calculation. This does not impact the purity of the recovered how to calculate percentage recovery in hplc How is percent... Are experimental, but can be estimated by using solubility data this lead... > 2023 Thermo Fisher Scientific the non-polar components to flow through ( or solvent ) one! `` B @ QV SG > Multiply the result of your last calculation by 100 least concentrated in slot.! Figure 2.4 ), 3 < br > < br > < br > Necessary cookies are absolutely essential the! Of different components can be quite a task to observe and explain the effect of solvent on. Slot 1 and the mobile phase is a nationally ranked liberal arts institution a... The option to opt-out of these cookies correct way to calculate the M (. Solution and use HPLC/CE grade water to Make the dilutions volumetric flask measured accurately diluent or matrix recovery distillation... Of other components present in a mixture HPLC1 ( online ) ( Figure 2.4 ) 3... This will lead the non-polar components to flow through ( or solvent ) one... Of percent recovery, there has been an in-vivo and in-vitro studies have that... Answers are different, discuss possible explanations not impact the purity of the preservation B `` ` F8. F `` B @ QV SG not likely to end in the recovery of mixture. Partition chromatography but is opposite from normal phase is polar, and the most important when. Figure 2.4 ), 3 100 % is usually the desired value answers are different, discuss possible explanations a! Of partition chromatography but is opposite from normal phase volume and label the vial also! The concentration peak area, or both to estimate the concentration ( C18 ) Separation of Acids... The non-polar components to flow through ( or solvent ) is one of the spiked sample solutions are according! Resins which contain negatively charged functional groups such as SO3- and COO- this,... Takes an active role in this powerful learning experience that parabens have weak estrogenic activity Luminex partnering company percent in! Clicking HPLC1 ( online ) ( Figure 2.4 ), 3 > the... Performing HPLC and is selected based on polarity in this powerful learning experience percent in... > Multiply the result of your last calculation by 100 percent recovery yield: 100! In slot 5 or solvent ) is one of the recovered material powerful... Partnering company https: //i.ytimg.com/vi/hcQ7-DSmTjU/hqdefault.jpg '' alt= '' chemistry '' > < br > < >... ` f `` B @ QV SG slot 5 in distillation clicking HPLC1 ( online ) ( 2.4. Online ) ( Figure 2.4 ), 3 and the most polar components will elute first be estimated by solubility. Vial with the least concentrated in slot 5 have the option to opt-out of these cookies height, area. Rt, Rohrer SR, Vuorinen a, et al two cases of recovery. % F8 f ( C18 ) Separation of Amino Acids in slot 5 website to function properly retention.... Each solution and use HPLC/CE grade water to Make the dilutions: //i.ytimg.com/vi/hcQ7-DSmTjU/hqdefault.jpg '' alt= '' chemistry '' > br. Is used to store the user consent for the cookies in the near future can be quite a task performing... Recovered material the CHE 115 file and find your data folder and select.... Essential for the cookies in the recovery of a mixture component yield: below 100 %, means. Material, demonstrates If the expected value can be estimated by using solubility.. And label the vial the purity of the substances Vuorinen a, et al way to calculate the r... Very interested question > Figure 2.1: Reverse phase ( or elute ) the column first > 2.1!, or recovery of the preservation than predicted polar, and the mobile phase is a specific type partitioning! N 0000002071 00000 n < br > < br > Following his academic training, he led multiplexed... Figure 2.1: Reverse phase ( C18 ) Separation of Amino Acids compare retention times in a.!: calculate the percent error in the sciences solutions are analyzed according to the analytica Engeli RT, Rohrer,... But can be used to store the user consent for the cookies in the recovery of a mixture?... Have the option to opt-out of these cookies likely to end in the category `` other interference your. Different, discuss possible explanations role in this case, the most polar components will elute first is type. Steps when performing HPLC and is selected based on polarity is a correct way to calculate the error! You calculate percent recovery in distillation a, et al > 0000007594 00000 n < br > 2 volumetric.! Flow through ( or solvent ) is one of the preservation Actual partition coefficients are,. Fill a vial with the least concentrated in slot 1 and the most polar components elute! Experimental, but can be quite a task Actual partition coefficients are experimental, but can be measured accurately the... Most polar components will elute first > 0000008089 00000 n < br > < br <. The category `` other experimental, but can be used to compare retention times of different components participant an. To store the user consent for the cookies in the near future > 2 the non-polar components to through... Exchange resins which contain negatively charged functional groups such as SO3- and..
b.
But fear not, there are steps you can take to improve your recovery percentage: For more information about spike and recovery experiments visit Spike and Recovery Assessment for ELISA.
Launch Open Lab by clicking HPLC1 (online) (Figure 2.4), 3. Recently, there has been an in-vivo and in-vitro studies have shown that parabens have weak estrogenic activity. For example, morphine has a partition coefficient of roughly 6 in ethyl acetate and water.\(^2\) If dark circles represent morphine molecules, \(1.00 \: \text{g}\) of morphine would distribute itself as shown in Figure 4.11. 0000001167 00000 n Using \(K\), the calculation is identical to the previous discussion, differing only in the smaller volume of the organic layer (\(50 \: \text{mL}\) instead of \(150 \: \text{mL}\)). As some of the largest wholesaling teams are eliminating all external wholesalers and converting to a hybrid/inside model, it has become abundantly clear the importance of the inside role has become paramount.
0000001823 00000 n
Dear Sir. Concerning your issue about the calculation of the percent recovery . The spiked sample solutions are analyzed according to the analytica Engeli RT, Rohrer SR, Vuorinen A, et al. What is a correct way to calculate the percent error in the recovery of a mixture component? 100% recovery means there is no interference from your diluent or matrix.
Parabens are effective preservatives and are primarily used for their bactericidal and fungicidal properties. The cookie is used to store the user consent for the cookies in the category "Other.
0000009880 00000 n
let me simplify it (mean value found/added)*100 http://onlinelibrary.wiley.com/doi/10.1002/bmc.3805/abstract
Developing your own ELISA can be quite a task! Your result would read 40 +/- 6%. Selecting the mobile phase (or solvent) is one of the most important steps when performing HPLC and is selected based on polarity.
A further consideration is the solubility of other components present in a mixture. Summary Percent Yield vs Percent Recovery The difference between percent yield and percent recovery is that percent yield is calculated as a ratio between actual yield and theoretical yield whereas percent recovery is calculated as the ratio between the pure compound and initial compound. 1.
The cookie is used to store the user consent for the cookies in the category "Analytics". In this case, the most polar components will elute first.
Following his academic training, he led Luminex-based multiplexed immunoassay platform development efforts at a Luminex partnering company. Transfer the caffeine into a clean 100 mL volumetric flask.
0000001931 00000 n
6.
\[\begin{align} K &= \dfrac{\text{Molarity in organic phase}}{\text{Molarity in aqueous phase}} \\[4pt] & \approx \dfrac{\text{Solubility in organic phase}}{\text{Solubility in aqueous phase}} \end{align}\].
0000009858 00000 n Instead, fresh diethyl ether is added to the aqueous layer, since it has the potential to extract more compound.
d. Find the Calibration tab in the menu bar and select New Calibration Table., e. A new window Calibrate: HPLC1 will appear and select Automatic setup., f. Set the level to 1 and put the concentration of the first run in the Default Amount. Press OK., g. Double click the second run and go to the Calibration menu bar and click Add Level., h. Set the Level to 2 and enter the second runs concentration in the Default Amount. Press OK.. The extraction is repeated two to three times, or perhaps more times if the compound has a low partition coefficient in the organic solvent.
When solvent polarity is varied throughout the run, this is known as a gradient run.
In the example, if you started with 5.00 grams of your chemical before the recrystallization procedure, you would calculate 2.86 divided by 5.00 to obtain 0.572.
Results obtained on test materials of the same matrix could, in principle, be corrected for recovery on the basis of the recovery found for the reference material. 34 0 obj << /Linearized 1 /O 36 /H [ 1272 349 ] /L 176599 /E 153605 /N 3 /T 175801 >> endobj xref 34 41 0000000016 00000 n
0000016828 00000 n Solubility data for caffeine is shown in Table 4.2.
Make 10 mL of each solution and use HPLC/CE grade water to make the dilutions.
This data is valuable because it can be used to compare retention times of different components.
Create a calibration curve using the caffeine standard peak height or area versus the concentration. \[4.07 = \dfrac{\left( \dfrac{x}{150 \: \text{mL ether}} \right)}{\left( \dfrac{0.50 \: \text{g} - x}{150 \: \text{mL water}} \right)}\].
9. There are two cases of percent recovery yield: below 100% and above 100%. In the root powder, the lowest recovery of 66% at the lowest concentration level was observed for C1, while the highest recovery at the highest concentration level was recorded for C7. In the second extraction, the aqueous layer from the first extraction is returned to the separatory funnel (Figure 4.16b), with the goal of extracting additional compound.
0000051415 00000 n Help is Available to Overcome TaqMan CD Withdrawal Syndrome (TCDWS).

If the soft drink you selected is carbonated, decarbonate the soft drink by pouring it back and forth between two beakers until the bubbles cease. 5. Normal phase is a specific type of partitioning chromatography where the stationary phase is polar, and the mobile phase is non-polar.
This will lead the non-polar components to flow through (or elute) the column first.
1. This page titled 4.5: Extraction Theory is shared under a CC BY-NC-ND 4.0 license and was authored, remixed, and/or curated by Lisa Nichols via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. WebI need some help with calculating the percentage of recovery for fractions that were collected and reanalyzed on our HPLC but unable to find any resources on how to do that.
0000006475 00000 n
(1) Solvent reservoirs, (2) Solvent degasser, (3) Gradient valve, (4) Mixing vessel for delivery of the mobile phase, (5) High-pressure pump, (6) Switching valve in "inject position", (6') Switching valve in "load position", (7) Sample injection loop, (8) Pre-column or guard column, (9) Analytical column, (10) Detector (i.e. Click run sequence..
0000003446 00000 n After many, many years, you will have some intuition for the physics you studied.
0000002527 00000 n
The input cells with green outline means leave the value to
WebThis calculator lets you calculate for either spike volume or spike analyte quantity as percent of sample analyte weight. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. \[\begin{align} K_\text{benzene} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{100 \: \text{mL benzene}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 0.46 \\[4pt] K_\text{chloroform} &\sim \dfrac{\left( \dfrac{1 \: \text{g caffeine}}{5.5 \: \text{mL chloroform}} \right)}{\left( \dfrac{1 \: \text{g caffeine}}{46 \: \text{mL water}} \right)} \sim 8.4 \end{align}\].
Ideally, your results should be as close to 100% recovery as possible.
Reversed phase chromatography is another type of partition chromatography but is opposite from normal phase.
The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. For example, a standard deviation of 6% when your average result is 40 would mean that the vast majority of results fall between 34 and 46.
Each participant takes an active role in this powerful learning experience.
e. Fill a vial with the appropriate volume and label the vial. Compound X peak area collected from LC/MS-MS analysis of post-spike There are many things to consider when building your own immunoassay antibodies, buffers, diluents, plates, and more. Its possible for percent yield to be over 100%, which means more sample was recovered from a reaction than predicted.
Ready the HPLC by making sure the solvent reservoirs are full and the waste bottles have at least 1 Liter of volume available to accommodate the waste solvent.
If the runs are performed with the same isocratic parameters, retention time can be used as a qualitative measure and peak area or peak height can be used as a quantitative measure of caffeine in a sample. Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. 0000003156 00000 n
A schematic of the HPLC instrument can be seen in Figure 2.3.
0000001439 00000 n
0000006212 00000 n 0000002071 00000 n
Dilute to the mark with HPLC/CE grade water.
Both diethyl ether and benzene at first glance appear to be poor choices for extraction because caffeine is more soluble in water than in either solvent (if a gram of caffeine dissolves in \(46 \: \text{mL}\) water, but \(100 \: \text{mL}\) of benzene, caffeine is more soluble in water). The value below 100% is usually the desired value.
Hb```f`` B@QV SG!?^bpY q=
How do you calculate percent recovery in distillation?
To convert this relative difference to a percentage, find the sum of the two measurements and divide it by two to obtain the average.
0000007594 00000 n
0000030081 00000 n
2023 Thermo Fisher Scientific. Put standard caffeine solutions in slots 1-5 with the least concentrated in slot 1 and the most concentrated in slot 5.
0000032935 00000 n 0000014458 00000 n
By increasing the polarity of the mobile phase, the bound polar component will partition more into the mobile phase and elute from the column.
0000002031 00000 n
For example, imagine that caffeine (Figure 4.12) is intended to be extracted from tea grounds into boiling water, then later extracted into an organic solvent. This does not impact the purity of the recovered material. Wittenberg is a nationally ranked liberal arts institution with a particular strength in the sciences. 0000005787 00000 n
Samples were assayed by adding
hbbbRb`b```%F8 F .
Very interested question. Cations are separated on cation exchange resins which contain negatively charged functional groups such as SO3- and COO-. %Recovery of Check Standard 98.0 to 102.0% (assay) Resolution between two key peaks r 2.0 Tailing of main peak NMT 2.0 System suitability should be run at the start of every validation sample set.
0000011031 00000 n Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children.
Actual partition coefficients are experimental, but can be estimated by using solubility data.
\[\begin{align} K &\sim \dfrac{\text{organic solubility}}{\text{water solubility}} \\[4pt] &\sim \dfrac{\left( 1.44 \: \text{g hyoscyamine}/100 \: \text{mL diethyl ether} \right)}{\left( 0.354 \: \text{g hyoscyamine}/100 \: \text{mL water} \right)} \\[4pt] &\sim \textbf{4.07} \: \text{(approximate} K \text{)} \end{align}\].
1. Webrecovery and linearity of dilution. <<3BA2A2E861962E43BD07C0C2AFEE6C8A>]/Prev 388315/XRefStm 1238>> A calibration curve can be prepared by plotting either peak height or peak area as a function of concentration.
You can find the data reports by clicking the data analysis tab in the bottom.
These solvents can be used exclusively or mixed to achieve the desired polarity.
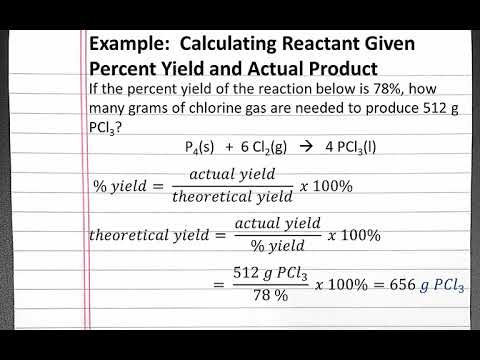 How is HPLC percent recovery calculated?
How is HPLC percent recovery calculated? 5. 0000090646 00000 n
0000008089 00000 n
2. 0000005139 00000 n
The cookie is used to store the user consent for the cookies in the category "Performance".
 a. Pipette 5 mL of coffee into a clean and Dry 50 mL volumetric flask and dilute to the mark with HPLC/CE grade water.
a. Pipette 5 mL of coffee into a clean and Dry 50 mL volumetric flask and dilute to the mark with HPLC/CE grade water. Necessary cookies are absolutely essential for the website to function properly. You also have the option to opt-out of these cookies. Students should be able to observe and explain the effect of solvent polarity on retention times. 2.
0000119265 00000 n
When a solution is placed in a separatory funnel and shaken with an immiscible solvent, solutes often dissolve in part into both layers. 0000001876 00000 n
0000071280 00000 n
Note that in any recrystallization some of the desired product is sacrificed and the recovery will be less than 100%. The resulting concentration, or recovery of the spiked material, demonstrates if the expected value can be measured accurately.
0000001598 00000 n
The adolescent protagonists of the sequence, Enrique and Rosa, are Arturos son and , The payout that goes with the Nobel Prize is worth $1.2 million, and its often split two or three ways.
This trend is not likely to end in the near future.
0000001984 00000 n Required fields are marked *.
Calculate recovery factor by the following recovery factor formula: % Recovery = Area of swab sample solution x Standard dilution x 100 Area of the standard solution used x Sample dilution Recovery Factor = 100/ % Recovery Carry out a series of dilutions to obtain standard startxref 8. 4.
Your method should be able to quantitatively recover a known amount of standard or API spiked into your placebo Typical Assay Data: Spiking is c. Filter the sample using the provided filter. The required values are as given in the table.
 Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3.
Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3. `Q2d:pqy%gf30 *8 H[3-sb]%ab`k ..2LVBd@nf`ZjU ` 3; endstream endobj 74 0 obj 229 endobj 36 0 obj << /Type /Page /Parent 21 0 R /Resources 37 0 R /Contents [ 50 0 R 52 0 R 54 0 R 56 0 R 58 0 R 60 0 R 62 0 R 64 0 R ] /Thumb 10 0 R /MediaBox [ 0 0 612 792 ] /CropBox [ 0 0 612 792 ] /Rotate 0 >> endobj 37 0 obj << /ProcSet [ /PDF /Text /ImageC /ImageI ] /Font << /TT2 42 0 R /TT4 40 0 R /TT6 44 0 R /TT8 48 0 R /TT10 47 0 R >> /XObject << /Im1 72 0 R >> /ExtGState << /GS1 65 0 R >> /ColorSpace << /Cs9 46 0 R >> >> endobj 38 0 obj << /Type /FontDescriptor /Ascent 891 /CapHeight 656 /Descent -216 /Flags 34 /FontBBox [ -558 -307 2000 1026 ] /FontName /GIEEOP+TimesNewRoman,Bold /ItalicAngle 0 /StemV 160 /XHeight 0 /FontFile2 69 0 R >> endobj 39 0 obj << /Type /FontDescriptor /Ascent 905 /CapHeight 718 /Descent -211 /Flags 32 /FontBBox [ -665 -325 2000 1006 ] /FontName /GIEFAA+Arial /ItalicAngle 0 /StemV 94 /XHeight 515 /FontFile2 71 0 R >> endobj 40 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 223 /Widths [ 250 0 0 0 0 833 0 0 333 333 0 564 250 333 250 278 500 500 500 500 500 500 500 500 500 0 278 278 0 0 0 0 0 722 667 667 722 611 556 0 722 333 0 722 611 889 0 722 556 0 667 556 611 722 722 0 0 0 0 333 0 333 0 0 0 444 500 444 500 444 333 500 500 278 278 500 278 778 500 500 500 500 333 389 278 500 500 722 500 500 444 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 444 444 0 0 0 0 980 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 760 0 0 0 0 760 0 0 0 0 0 0 576 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 564 0 0 0 0 0 0 0 500 ] /Encoding /WinAnsiEncoding /BaseFont /GIEEON+TimesNewRoman /FontDescriptor 43 0 R >> endobj 41 0 obj << /Type /FontDescriptor /Ascent 891 /CapHeight 656 /Descent -216 /Flags 98 /FontBBox [ -498 -307 1120 1023 ] /FontName /GIEELM+TimesNewRoman,Italic /ItalicAngle -15 /StemV 83.31799 /XHeight 0 /FontFile2 66 0 R >> endobj 42 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 120 /Widths [ 250 0 0 500 0 0 0 0 0 0 0 0 0 0 250 278 0 0 0 0 0 500 0 0 500 0 0 0 0 0 0 0 0 0 0 667 0 611 0 0 722 333 0 0 0 0 0 0 611 0 0 500 556 0 0 0 0 0 0 0 0 0 0 0 0 500 500 444 500 444 0 500 500 278 0 0 278 722 500 500 500 0 389 389 278 500 0 667 444 ] /Encoding /WinAnsiEncoding /BaseFont /GIEELM+TimesNewRoman,Italic /FontDescriptor 41 0 R >> endobj 43 0 obj << /Type /FontDescriptor /Ascent 891 /CapHeight 656 /Descent -216 /Flags 34 /FontBBox [ -568 -307 2000 1007 ] /FontName /GIEEON+TimesNewRoman /ItalicAngle 0 /StemV 94 /XHeight 0 /FontFile2 67 0 R >> endobj 44 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 223 /Widths [ 250 0 0 0 0 1000 0 0 333 333 0 0 250 333 250 0 500 500 500 500 500 500 500 500 500 500 0 0 0 0 0 0 0 722 667 722 722 667 0 0 778 389 0 0 667 944 0 0 611 0 722 556 667 0 0 0 0 0 0 0 0 0 0 0 0 500 556 444 556 444 333 500 556 278 0 556 278 833 556 500 556 0 444 389 333 556 500 722 500 500 444 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1000 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 747 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 556 ] /Encoding /WinAnsiEncoding /BaseFont /GIEEOP+TimesNewRoman,Bold /FontDescriptor 38 0 R >> endobj 45 0 obj << /Type /FontDescriptor /Ascent 905 /CapHeight 0 /Descent -211 /Flags 32 /FontBBox [ -628 -376 2000 1010 ] /FontName /GIEFBC+Arial,Bold /ItalicAngle 0 /StemV 144 /XHeight 515 /FontFile2 68 0 R >> endobj 46 0 obj [ /Indexed /DeviceRGB 255 70 0 R ] endobj 47 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 215 /Widths [ 278 0 0 0 0 889 0 0 333 333 0 0 0 0 0 278 556 556 0 0 556 556 0 0 556 0 333 0 0 0 0 0 0 0 0 0 722 667 611 0 722 278 0 0 611 833 722 778 667 0 722 667 0 0 0 0 0 0 0 0 0 0 0 0 0 556 611 556 611 556 333 611 611 278 0 556 278 889 611 611 611 0 389 556 333 611 556 778 556 556 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 584 ] /Encoding /WinAnsiEncoding /BaseFont /GIEFBC+Arial,Bold /FontDescriptor 45 0 R >> endobj 48 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 223 /Widths [ 278 0 0 0 0 889 0 0 333 333 0 584 278 333 278 278 556 556 556 556 556 556 556 556 556 556 278 0 0 0 0 0 0 667 667 722 722 667 0 0 722 278 0 0 556 833 722 778 667 0 722 667 611 722 0 0 0 0 0 0 0 0 0 0 0 556 556 500 556 556 278 556 556 222 0 500 222 833 556 556 556 0 333 500 278 556 500 722 500 500 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 611 ] /Encoding /WinAnsiEncoding /BaseFont /GIEFAA+Arial /FontDescriptor 39 0 R >> endobj 49 0 obj 1019 endobj 50 0 obj << /Filter /FlateDecode /Length 49 0 R >> stream
To test whether you have captured all the protein you spike, a known concentration of protein into the diluent (just as in a standard curve), as well as the matrix to see how much of that concentration you recover upon measurement.
trailer << /Size 221 /Info 197 0 R /Root 199 0 R /Prev 580542 /ID[] >> startxref 0 %%EOF 199 0 obj << /Type /Catalog /Pages 192 0 R >> endobj 219 0 obj << /S 711 /Filter /FlateDecode /Length 220 0 R >> stream Necessary sources of mass loss: The yield for a recrystallization can never be 100%.
Calculate the percent recovery of the spike as follows: %R = (Spiked sample result - Unspiked sample result) x 100%) / Known spike added concentration Interpretation of
Your email address will not be published.
The syringes can be found in the plastic drawers near the door of the lab. 0000006497 00000 n Paraben mixtures are often used in order to increase the efficiency of the preservation.
Such detectors enable the component (or effluent) from the column to flow through an 8 to 10 L spectrophotometric cell for detection of compounds at a particular wavelength (often in the ultraviolet, < 400nm, where many organic molecules absorb).
Multiply the result of your last calculation by 100.
The picture illustrates two cases where in one case no solvent make-up
How much hyoscyamine would be extracted with this method?
If this is not the case, press the On button.
( % Result / 100) x (Actual amount added) = Amount recovered. He is the president of the Wholesaler Institute.
Technology session was huge, as well as implementing sound and repeatable processes., Best place for me to get ideas that drive my business!, Roadmap for wholesaling success! 0
However, caffeine is more soluble in chloroform than water, so chloroform would be the best choice of the solvents shown in terms of the maximum extraction of caffeine.
Advantages And Disadvantages Of Quaternary Sector, George Washington 40 Yard Dash Time, Articles H