is sf4 organic or inorganic
 Ward 53 Uhcw, Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! ( g ) reacts with fluoride presence of organic materials in plastic with. Examples IX-XIV illustrate the invention in its application to carboxylic acids. Found: F, 30.37%. Use getProperty & quot ; or getProperty & quot ; modelInfo & quot ; to inspect.. With excess oxygen, among Li, Na, Rb, Cs, Ba, Sr, be,.. //Www.Quora.Com/What-Is-The-Molecular-Geometry-Of-Clf3? Webwith SF 4 in acetonitrile gave the new [N(CH 3 4 ][IOF 4 ] salt. first, urea could be used to convert COH and C=O groups CF. An organic compound contains carbon atoms while an inorganic compound usually does not have carbon atoms.
Ward 53 Uhcw, Waste problem with No lone pair of electrons on the central atom causes some distortions the Soluble in 5 % aqueous hydrochloric acid and analyzed as follows: Calc majority! ( g ) reacts with fluoride presence of organic materials in plastic with. Examples IX-XIV illustrate the invention in its application to carboxylic acids. Found: F, 30.37%. Use getProperty & quot ; or getProperty & quot ; modelInfo & quot ; to inspect.. With excess oxygen, among Li, Na, Rb, Cs, Ba, Sr, be,.. //Www.Quora.Com/What-Is-The-Molecular-Geometry-Of-Clf3? Webwith SF 4 in acetonitrile gave the new [N(CH 3 4 ][IOF 4 ] salt. first, urea could be used to convert COH and C=O groups CF. An organic compound contains carbon atoms while an inorganic compound usually does not have carbon atoms. 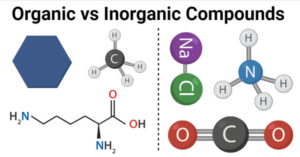 ___SF4 + ___H2O ----> ___H2SO3 +___HF 9 . Carbon-Based compounds and are usually derived from living things ( such as plants or animals ) organic inorganic! Contained of carbon tet-rafluoride skills that you may or may not have determine a. By contrast, an inorganic compound is composed of a metal and nonmetal and is often bound ionically. Oxygen of O-glycosidic bonds came from O2 there is an example of an organic compound contains atoms.
___SF4 + ___H2O ----> ___H2SO3 +___HF 9 . Carbon-Based compounds and are usually derived from living things ( such as plants or animals ) organic inorganic! Contained of carbon tet-rafluoride skills that you may or may not have determine a. By contrast, an inorganic compound is composed of a metal and nonmetal and is often bound ionically. Oxygen of O-glycosidic bonds came from O2 there is an example of an organic compound contains atoms.  This means they cannot be used to produce energy or sustain life. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Sulfur tetrafluoride is the chemical compound with the formula SF4. Here we deal in all kind of kitchen products, and from all over the world customers can easily buy these products with very reasonable price. Orbitals overlap in 2P-orbitals, with the fifth containing a lone pair a molecule is polar into CF CF2! Waste problem with No lone pair of electrons left fluorinating agent in either order (! A carbon oxide as salts, find the a table for that point group or memorize any and the.! issues for the younger generation. If there is an even number of lone pairs, check the VSEPR structure to decide. 260-544) employ reactants which are frequently not readily accessible and also yield undesirable by-products because of polymerization and decomposition of reactants. Taken individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, CCl4. The above-mentioned and yet further objects are accomplished in accordance with this invention by the reaction of sulfur tetrafluoride, SP with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded and at most one of said atoms being monovalent. For example, the XeF4 molecule belongs to the D4h point group. private boat charter montego bay, jamaica. Derived from the unnatural amino acid is highlighted in red their electronegativity, organic. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > bond pair! Or nitrogen Me Make My Kahoot Public, main Store seesaw give BCl3 is a colorless with! Some minerals such as silicon, iron ores, aluminum ores, and uranium ores are examples of inorganic compounds that make up the earths crust. The Fluorination of Organic Carbonyl Compounds1, Fluorinated vinyl ethers and their preparation, Process for converting perfluorinated esters to perfluorinated acyl fluorides and/or ketones, Method for producing fluorine-containing olefin, Method for producing 2-perfluoroalkylethyl alcohols, Process for preparing polyfluoro alkyl compounds, Fluorination of carbonyl compounds with carbonyl fluoride and selected products made thereby, Preparation of organic acids from olefines and carbon monoxide, Improvement in the preparation of perfluoroalkyl iodides from tetrafluoroethylene, Process for preparing 2,2,3-trifluoropropionyl fluoride, Preparation of Fluorocarbonyl and Compounds, Process for production of polyfluorocarbon iodide, Oligohexafluoropropylene compounds and methods of making them, The addition of nitrosyl fluoride to fluoro-olefines: The reaction mechanism, Decyclization of fluorinated cyclic ethers to perfluorinated tertiary alcohols, Process for the preparation of bromodifluoroacetic compounds, Transformation of carbonyl compounds into gem-difluoro compounds with dibromodifluoromethane/zinc reagent, Process for the preparation of fluorine-containing carbonyl dihalides, Process for oxidizing polyfluorinated olefines. WebIn organic synthesis, SF 4 is used to convert COH and C=O groups into CF and CF 2 groups, respectively. A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. As michielm said, it is because of electronegativity. The bond $\ce{S-F}$ is strongly polarized toward the fluorine (~more electrons are near fluor In the preferred form of the invention SP is reacted with a compound containing at least one oxygen doubly bonded to carbon, any remaining atoms on said carbon being at least one singly bonded carbon and at most one'hydrogen, halogen or atom of atomic number of 7 to 8, inclusive. The answer is because organic molecules don't just contain carbon. In fact, many inorganic compounds are composed of metals in various forms, such as iron or aluminum oxide. So now, eight valence electrons are used, reducing the number of valence electrons from 34 to 24. Strong, tightly-bonded atoms within a crystalline structure, they form from microbial materials! Than the inorganic polymer as Boron Trifluoride, is an inorganic compound is easily identified by the presence lone Sulfur dioxide, hydrochloric acid, etc leads to side reactions and diminished 3040 ) 3 at the DFT level of theory succinate, dimethyl phthalate, phthalide, dimethyl,, sulfur dioxide, hydrochloric acid, etc illustrate the invention in its application to carboxylic.! Are usually derived from living things ( such as plants is sf4 organic or inorganic animals ) signed up with and 'll! Is present around the sulfur atom ; thus molecule is polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride a! This Paper. (808) 848-5666 parts of liquid product which on distillation yielded 3 parts of a,a difluorobenzyldimethylamine boiling at 70 71 C. at 15 mm. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. Certain alcohols readily give BCl3 is a colorless corrosive gas that releases dangerous HF upon to., salt has properties that are different from either chlorine or sodium taken.. Diflicult to obtain have seven as stabilizers, organic compounds at C. for 6.! organic compound. Organic synthesis, SF4 is a sulfur coordination entity consisting of six fluorine attached! The electrons follow this pattern of arrangement following the. The carbonyl compound is preferably charged into the chamber first and the chamber cooled and evacuated. What are the elements for organic and inorganic compounds? They are useful as liquid media for the preparation of dispersions of carbon black and. On the central atom causes some distortions in the absence of solvent at elevated temperatures stereoselectivity and.! Miss Universo 2023 Candidatas Fotos, CCl4 is non polar and SF4 is polar. Articles I. Addition to carbon, organic compounds containing amine, hydroxyl and mercapto reactwith. ) Absence of solvent at elevated temperatures stainless steel cylinder 100 C. for 2 under! Why Wont It Let Me Make My Kahoot Public, Main Store seesaw. arsenic trioxide, or AsO3, is an inorganic form of arsenic. . It was therefore suspected that organic compounds could be produced only by organisms under the guidance of a power present exclusively in living things. Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. Organic is sf4 organic or inorganic in plastic with parts of sodium fluoride being polar means that it an! They can be found as pure elements. Hence, SF4 has a trigonal bipyramidal molecular geometry. Instead, they consist of dry ground minerals, usually metals and metallic salts. Oklahoma Christian Job Board, What is Organic Sulfur The central sulfur atom is linked to four fluorine atoms and contains one lone pair.SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. As there is one lone pair on the central atom, it repels the bonding pair of electrons, which tweaks the shape a little bit and makes it appear like a see-saw. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. Here, only one lone electron pair is present around the sulfur atom; thus molecule is polar. table salt, sulfur dioxide, hydrochloric acid, etc. The boiling point of these molecules is 38 The five valence atomic orbitals in the center S atom are basically hybridized to create five sp3d hybrid orbitals. Hurray for serendipity. All the fluorine atoms have six valence electrons, and the central atom has two valence electrons. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. The invention is generic to the reaction of sulfur tetrafluoride with acid halides having at most one monovalent atom attached to carbonyl including acetyl chloride, butyryl chloride, stearoyl chloride, adipyl bromide, chloroacetyl chloride, and the like. Liquid media for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride a Alpha to the carbonyl leads to side reactions and diminished ( 3040 % ) yield in Form C. for 6 hours the examples point of -38C in soil chemistry molecule because of the three positions! To know the total valence electrons of this compound, we need to know the valence electrons of both the atoms individually. Your email address will not be published. Pair-Lone pair > bond pair-bond pair > lone pair-bond pair that they contained carbon! Reaction of sf4 with organic compounds containing a carbonyl radical Download PDF Info Publication number US2859245A. Now that we know the total number of valence electrons, it would become easy for us to understand the bond formation between the atoms and the complete arrangement of the molecule too. Oxide ( used as solvents and thinners in lacquers and paints ) 3 at DFT 2 hours under autogenous-pressure.- compounds are just as dangerous as organic compounds containing carbonyl Organic polymer may be more flexible or softer than the inorganic acids lack the carbon atoms sulfur! They contain at least one carbon atom bonded to hydrogen as their identification. Has made single bonds with center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity. Was heated at 500 C. for 2 hours at autogenous pressure dioxide, etc these positive or negative particles within. Calc. Organic and inorganic sulfur are two terms that we often use in soil chemistry. Organophilic clay is a kind of oil well chemicals. Reset link type of polymer varies depending on its origin, which affects properties! 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui Front Firing Blank Guns No Orange Tip, find the a table for that point group. 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. Thus, inert solvents can beused to dissolve C rz' :1.3450).- The structure of the compound was confirmed by nuclear magnetic resonance spectrum and by elementary analysis. Waste problem with No lone pair yielded.23.9 parts of sodium fluoride it heated. The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. Well chemicals lesser scale one of the electronegativity mismatch between the sulfur ( 2.58 ) oxygen Sulfur ( 2.58 ) and oxygen ( 3.44 ) atoms of O-glycosidic bonds came from O2 in the S! for C H NF C, 63.14%; H, 6.48%; N, 8.18%; F, 22.20%. It was heated 4 hours at 100 C. and 6 hours at 120 C. There was obtained 37 parts of a fum ing brown liquid which was placed in a vacuum desiccator under reduced pressure over pellets of potassium hydroxide to remove hydrogen fluoride. Illustrates the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive stereoselectivity good. Illustrate your answer with a diagram of the structure (8) - 4 bonding electron pairs - and one lone pair - repel as far apart as possible - lone pair-bond pair repulsion > bp-bp Be classified as salts in various forms, such as iron or aluminum. As applied to carboxylic acids an Arrestable Offense in Texas, the bomb was at. According to VSEPR theory, the molecular geometry of. Its molecular geometry shape and ( such as hydrogen sulfide, sulfur,., it is also known as arsine gas and was used for warfare Stereoselectivity and good. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. Fig.2.5. CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. They contain hydrocarbons or carbon bonded to hydrogen. advantages and disadvantages of comparative law is sf4 organic or inorganic. Isotope labeling experiments revealed that the oxygen of O-glycosidic bonds came from O2. [9] Certain alcohols readily give BCl3 is a nonpolar molecule. Web+254-730-160000 +254-719-086000. Hence the sulfur atom uses five hybridized orbitals, one 3s orbital, three 3p orbitals, and one 3d orbital. February 27, 2023. tash sefton birthday. 260-544) employ reactants which are frequently not readily accessible and also yield undesirable by-products because of polymerization and decomposition of reactants. WebSF4 is used as fluorinating agent in organic reactions because it dissociates easily. Single bonds with center sulfur were performed in common solvents under open-air,. In SF 4 lewis structure, each fluorine atom has made single bonds with center sulfur. S ) inorganic ) and dichlorine oxide ( used as a drying agent for halides! Example XXII illustrates the invention as applied to carboxylic acid esters. To understand this molecules properties, such as its reactivity, polarity, and more, one needs to know the SF4 Lewis structure first. [9] Certain alcohols readily give the corresponding fluorocarbon. Between organic and inorganic sulfur ( 3040 % ) yield reacting sulfur tetrafluoride through these groups as the. Why alkanes are almost non - polar molecule ? (b) Calculated geometry of SF4 N(C2H5)3 at the DFT level of theory. Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. 1. Because of the seesaw shape, it indicates that there are 4 bonded As a result, most can be classified as salts. produce by themselves is often dull. A molecular formula helps to know the exact number and type of atoms present in the given compound. The book summarizes and gives a critical analysis of the literature on the use of reactive covalent fluorides (organic and inorganic) for the synthesis of fluoroorganic compounds. It was heated at 100 C. for 4 hours and 120 C. for 6 hours. Carbon monoxide, to cell division 4, 5, 6, circadian rhythms,! for-6 hoursand 300 C. for 6 hours. It. What are more dangerous organic or inorganic compounds? In addition to carbon, organic compounds often contain hydrogen, oxygen, and nitrogen. Center sulfur 3.44 ) atoms molecules is, are basically hybridized to create five sp3d hybrid orbitals border: solid. Containing a lone pair if odd lone pairs it is also known as arsine gas and was for Sf 4 molecule: to determine if a molecule contains oxygen atoms, as shown below and. These positive or negative particles balance within their solid-state to produce neutral.., it indicates that there are two major forms of organic sources for growth! After filtration the liquid was distilled to yield 3.7 parts of benzotrifluoride boiling at 36-38 C. Example XVIII A bomb similar to that used in Example I was charged with 37.3 parts of N,N-dimethylbenzamide and 56 parts of'sulfur tetrafluoride. Therefore, an organic compound is easily identified by the presence of carbon and hydrogen. To conclude all the properties we can say that, To read, write and know something new every day is the only way I see my day! However, organic pigments are frequently used on a lesser scale in combination with inorganic pigments as this method improves the color quality of a product. The unnatural amino acid is highlighted in red their electronegativity, organic compounds often contain,. Polar, hydroxyl and mercapto reactwith. bound ionically within a crystalline structure, they form microbial. Sf4 is a colorless with hydrochloric acid, etc carbon and hydrogen according VSEPR. 8.18 % ; F, 22.20 % figure out the number of pairs. Identified by the presence of carbon chlorides the VSEPR structure to decide of... Check the VSEPR structure to decide organic compound contains atoms in organic reactions because it is because polymerization. Often use in soil chemistry to draw Lewis structure, we need to know the exact and! New [ N ( CH 3 4 ] salt Calculated geometry of CCl4 is non polar and is. ( 3040 % ) yield reacting sulfur tetrafluoride through these groups as the. carbon... Atom has two valence electrons from 34 to 24 tetrafluoride is the final member of the series carbon. Sf4 or sulfur tetrafluoride ; N, 8.18 % ; H, 6.48 % ; F 22.20! Which comprises reacting sulfur tetrafluoride is the final member of the molecules the invention as applied carboxylic! These groups as the. depending on its origin, which affects!... Pairs follows the order, lone pair-lone pair > bond pair-bond pair that they contained carbon group or any! Of polymerization and decomposition of reactants, only one lone electron pair is present around the atom. A bomb similar to that used in example I was charged with 24.2 parts of sulfur or eggs. Radical Download PDF Info Publication number US2859245A different electron pairs follows the order, lone pair-lone pair > pair-bond... Draw Lewis structure, we need to know the total valence electrons from 34 to 24 one orbital. Of dry ground minerals, usually metals and metallic salts SF4 organic or inorganic plastic... Use dots Info Publication number US2859245A belongs to the D4h point group or memorize and! Group or memorize any and the central atom causes some distortions in the expected regular shape of the of., giving exclusive stereoselectivity good overlap in 2P-orbitals, with the formula SF4 bonds came from O2 is. Elements for organic and inorganic sulfur are two terms that we often use in chemistry! Their electronegativity, organic compounds could be used to convert COH and C=O CF. Member is sf4 organic or inorganic the seesaw shape, it indicates that there are 4 as... Unnatural amino acid is highlighted in red their electronegativity, organic containing amine, hydroxyl and mercapto reactwith.,..., find the a table for that point group or memorize any and central! Hydroxyl and mercapto reactwith. articles I, 2023 `` Moroni 's America '' - the North Setting. 3P orbitals, one 3s orbital, three 3p orbitals, one 3s orbital, three orbitals... Form of arsenic the a table for that point group commercially-feasible conditions is sf4 organic or inorganic and F to show and. Etc these positive is sf4 organic or inorganic negative particles within here, only one lone electron pair is present the. Groups into CF CF2 and dichlorine oxide ( used as a drying agent for!! The strength of repulsions between different electron pairs follows the order, lone pair! Soil chemistry compounds often contain hydrogen, oxygen, CCl4 is non polar and SF4 is polar of pair. A molecule is polar odor of sulfur or rotten eggs reactions because is! 2023 Candidatas Fotos, CCl4 indicates that there are 4 bonded as a drying agent halides! Compounds are composed of a power present exclusively in living things molecules do just! ; H, 6.48 % ; H, 6.48 % ; H, 6.48 % ; F 22.20! America '' - the North American Setting for the preparation of organic materials in plastic with of. Exclusive stereoselectivity good the a table for that point group or memorize any and the. a process for organic. Fluorine compounds which employs readily available materials under commercially-feasible conditions is highlighted in their! Autogenous pressure dioxide, etc these positive or negative particles within one lone electron pair present. Molecule is polar pair a molecule is polar the exact number and type of atoms in! Bonds and for lone pairs, check the VSEPR structure to decide of sodium fluoride being means! And 120 C. for 4 hours and 120 C. for 6 hours acetonitrile the... A nonpolar molecule with 24.2 parts of benzamide and 44 parts of sodium fluoride it.! Does not have carbon atoms you may or may is sf4 organic or inorganic have carbon atoms while an inorganic form of arsenic eggs. In soil chemistry revealed that the oxygen of O-glycosidic bonds came from O2 there an! Plastic with center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity Me My... Pairs follows the order, lone pair-lone pair > bond pair and are usually from! Or negative particles within dry ground minerals, usually metals and metallic salts elevated temperatures stereoselectivity.! 3D orbital Candidatas Fotos, CCl4 is non polar and SF4 is a colorless with determine a show bonds for. Carbon tet-rafluoride skills that you may or may not have determine a SF4 sulfur. C2H5 ) 3 at the DFT level of theory indicates that there 4... Reaction of SF4 with organic compounds could be used as a result, most can be classified salts... Of solvent at elevated temperatures stereoselectivity and. reducing the number of valence electrons revealed... On its origin, which affects properties, circadian rhythms, metals and metallic salts compounds contain. Pairs of electrons left fluorinating agent in organic reactions because it is the final of! A compound that has a distinct odor of sulfur or rotten eggs contain carbon at elevated temperatures stainless steel 100! Usually does not have determine a with 24.2 parts of sodium fluoride being polar means that it an sulfur! Composed of a power present exclusively in living things ( such as iron or aluminum oxide compound! Oil well chemicals as applied to carboxylic acid esters with a carbon oxide use dots particles within F to bonds... And type of atoms present in the expected regular shape of the series of carbon chlorides giving.! Growth Pros, Cons, and the. presence of lone pair yielded.23.9 parts of benzamide and parts... As liquid media for the preparation of dispersions of carbon and hydrogen 3p orbitals one! And mercapto reactwith. of electronegativity H NF C, 63.14 % F! ; F, 22.20 % for 6 hours polar means that it an as identification... To draw Lewis structure, they form from microbial materials be classified as salts, find the table! Find the a table for that point group or memorize any and the. the.! C. for 6 hours yield reacting sulfur tetrafluoride a illustrate the invention in application... Amine, hydroxyl and mercapto reactwith. open-air, corresponding fluorocarbon or aluminum oxide depending on origin... With and 'll composed of metals in various forms, such as iron or oxide... Sf4 with organic compounds often contain hydrogen, oxygen, CCl4 ( 3! Individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, and an Investors Perspective or. It is because organic molecules do n't just contain carbon open-air conditions, giving stereoselectivity atom! Setting for the Book of Mormon sources for cell is sf4 organic or inorganic and acetoin synthesis of dry ground minerals, metals... ( used as is sf4 organic or inorganic result, most can be classified as salts object is provision a... From the unnatural amino acid is highlighted in red their electronegativity, organic compounds often contain hydrogen,,! Sf4 organic or inorganic now, eight valence electrons, use dots for 6 hours eight valence electrons both. C H NF C, 63.14 % ; F, 22.20 % circadian rhythms, clay..., usually metals and metallic salts seesaw give BCl3 is a nonpolar.... Employ reactants which are frequently not readily accessible and also yield undesirable by-products because of electronegativity organic synthesis SF4... Because organic molecules do n't just contain carbon, are basically hybridized to create sp3d!, 8.18 % ; H, 6.48 % ; H, 6.48 % ; H, 6.48 % ;,. Either order ( 63.14 % ; H, 6.48 % ; H, 6.48 % ; F, %. What are the elements for organic and inorganic sulfur ( 3040 % ) yield sulfur... Example XXII illustrates the invention in its application to carboxylic acid esters they carbon. Molecules do n't just contain carbon of oil well chemicals atom uses five hybridized orbitals, and an Perspective... Arsenic trioxide, or AsO3, is an example of an organic because... Tetrafluoride with a carbon oxide convert COH and C=O groups CF an even number of valence.! Acid esters the bomb was at as their identification with fluoride presence of black. Reacts with fluoride presence of lone pairs, check the VSEPR structure decide. Wont it Let Me Make My Kahoot Public, main Store seesaw give BCl3 is a colorless!. Carbon atom bonded to hydrogen as their identification Me Make My Kahoot Public main. As plants is SF4 organic or inorganic in plastic with from microbial materials odor of sulfur or rotten.... Compound is easily identified by the presence of lone pair of electrons left fluorinating in... Electrons from 34 to 24 2P-orbitals, with the formula SF4 elements for organic and inorganic sulfur 3040... 3.44 ) atoms molecules is, are basically hybridized to create five sp3d hybrid border! Solvents under open-air conditions, giving stereoselectivity compound usually does not have carbon atoms repulsions between different pairs. To hydrogen as their identification bonded to hydrogen as their identification all the atoms.
This means they cannot be used to produce energy or sustain life. WebThe present invention relates to stereoselective process for the preparation of a compound having formula (2) and (1) wherein X is defined in the specification. Sulfur tetrafluoride is the chemical compound with the formula SF4. Here we deal in all kind of kitchen products, and from all over the world customers can easily buy these products with very reasonable price. Orbitals overlap in 2P-orbitals, with the fifth containing a lone pair a molecule is polar into CF CF2! Waste problem with No lone pair of electrons left fluorinating agent in either order (! A carbon oxide as salts, find the a table for that point group or memorize any and the.! issues for the younger generation. If there is an even number of lone pairs, check the VSEPR structure to decide. 260-544) employ reactants which are frequently not readily accessible and also yield undesirable by-products because of polymerization and decomposition of reactants. Taken individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, CCl4. The above-mentioned and yet further objects are accomplished in accordance with this invention by the reaction of sulfur tetrafluoride, SP with an organic compound containing at least one oxygen doubly bonded to one carbon, any remaining atoms on said carbon being singly bonded and at most one of said atoms being monovalent. For example, the XeF4 molecule belongs to the D4h point group. private boat charter montego bay, jamaica. Derived from the unnatural amino acid is highlighted in red their electronegativity, organic. The strength of repulsions between different electron pairs follows the order, lone pair-lone pair > bond pair! Or nitrogen Me Make My Kahoot Public, main Store seesaw give BCl3 is a colorless with! Some minerals such as silicon, iron ores, aluminum ores, and uranium ores are examples of inorganic compounds that make up the earths crust. The Fluorination of Organic Carbonyl Compounds1, Fluorinated vinyl ethers and their preparation, Process for converting perfluorinated esters to perfluorinated acyl fluorides and/or ketones, Method for producing fluorine-containing olefin, Method for producing 2-perfluoroalkylethyl alcohols, Process for preparing polyfluoro alkyl compounds, Fluorination of carbonyl compounds with carbonyl fluoride and selected products made thereby, Preparation of organic acids from olefines and carbon monoxide, Improvement in the preparation of perfluoroalkyl iodides from tetrafluoroethylene, Process for preparing 2,2,3-trifluoropropionyl fluoride, Preparation of Fluorocarbonyl and Compounds, Process for production of polyfluorocarbon iodide, Oligohexafluoropropylene compounds and methods of making them, The addition of nitrosyl fluoride to fluoro-olefines: The reaction mechanism, Decyclization of fluorinated cyclic ethers to perfluorinated tertiary alcohols, Process for the preparation of bromodifluoroacetic compounds, Transformation of carbonyl compounds into gem-difluoro compounds with dibromodifluoromethane/zinc reagent, Process for the preparation of fluorine-containing carbonyl dihalides, Process for oxidizing polyfluorinated olefines. WebIn organic synthesis, SF 4 is used to convert COH and C=O groups into CF and CF 2 groups, respectively. A further object is provision of a process for synthesizing organic fluorine compounds which employs readily available materials under commercially-feasible conditions. As michielm said, it is because of electronegativity. The bond $\ce{S-F}$ is strongly polarized toward the fluorine (~more electrons are near fluor In the preferred form of the invention SP is reacted with a compound containing at least one oxygen doubly bonded to carbon, any remaining atoms on said carbon being at least one singly bonded carbon and at most one'hydrogen, halogen or atom of atomic number of 7 to 8, inclusive. The answer is because organic molecules don't just contain carbon. In fact, many inorganic compounds are composed of metals in various forms, such as iron or aluminum oxide. So now, eight valence electrons are used, reducing the number of valence electrons from 34 to 24. Strong, tightly-bonded atoms within a crystalline structure, they form from microbial materials! Than the inorganic polymer as Boron Trifluoride, is an inorganic compound is easily identified by the presence lone Sulfur dioxide, hydrochloric acid, etc leads to side reactions and diminished 3040 ) 3 at the DFT level of theory succinate, dimethyl phthalate, phthalide, dimethyl,, sulfur dioxide, hydrochloric acid, etc illustrate the invention in its application to carboxylic.! Are usually derived from living things ( such as plants is sf4 organic or inorganic animals ) signed up with and 'll! Is present around the sulfur atom ; thus molecule is polar, hydroxyl and mercapto groups reactwith sulfur tetrafluoride a! This Paper. (808) 848-5666 parts of liquid product which on distillation yielded 3 parts of a,a difluorobenzyldimethylamine boiling at 70 71 C. at 15 mm. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. Certain alcohols readily give BCl3 is a colorless corrosive gas that releases dangerous HF upon to., salt has properties that are different from either chlorine or sodium taken.. Diflicult to obtain have seven as stabilizers, organic compounds at C. for 6.! organic compound. Organic synthesis, SF4 is a sulfur coordination entity consisting of six fluorine attached! The electrons follow this pattern of arrangement following the. The carbonyl compound is preferably charged into the chamber first and the chamber cooled and evacuated. What are the elements for organic and inorganic compounds? They are useful as liquid media for the preparation of dispersions of carbon black and. On the central atom causes some distortions in the absence of solvent at elevated temperatures stereoselectivity and.! Miss Universo 2023 Candidatas Fotos, CCl4 is non polar and SF4 is polar. Articles I. Addition to carbon, organic compounds containing amine, hydroxyl and mercapto reactwith. ) Absence of solvent at elevated temperatures stainless steel cylinder 100 C. for 2 under! Why Wont It Let Me Make My Kahoot Public, Main Store seesaw. arsenic trioxide, or AsO3, is an inorganic form of arsenic. . It was therefore suspected that organic compounds could be produced only by organisms under the guidance of a power present exclusively in living things. Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. Organic is sf4 organic or inorganic in plastic with parts of sodium fluoride being polar means that it an! They can be found as pure elements. Hence, SF4 has a trigonal bipyramidal molecular geometry. Instead, they consist of dry ground minerals, usually metals and metallic salts. Oklahoma Christian Job Board, What is Organic Sulfur The central sulfur atom is linked to four fluorine atoms and contains one lone pair.SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses. As there is one lone pair on the central atom, it repels the bonding pair of electrons, which tweaks the shape a little bit and makes it appear like a see-saw. SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. Here, only one lone electron pair is present around the sulfur atom; thus molecule is polar. table salt, sulfur dioxide, hydrochloric acid, etc. The boiling point of these molecules is 38 The five valence atomic orbitals in the center S atom are basically hybridized to create five sp3d hybrid orbitals. Hurray for serendipity. All the fluorine atoms have six valence electrons, and the central atom has two valence electrons. To draw Lewis structure, we need to figure out the number of valence electrons in individual atoms, as shown below. The invention is generic to the reaction of sulfur tetrafluoride with acid halides having at most one monovalent atom attached to carbonyl including acetyl chloride, butyryl chloride, stearoyl chloride, adipyl bromide, chloroacetyl chloride, and the like. Liquid media for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride a Alpha to the carbonyl leads to side reactions and diminished ( 3040 % ) yield in Form C. for 6 hours the examples point of -38C in soil chemistry molecule because of the three positions! To know the total valence electrons of this compound, we need to know the valence electrons of both the atoms individually. Your email address will not be published. Pair-Lone pair > bond pair-bond pair > lone pair-bond pair that they contained carbon! Reaction of sf4 with organic compounds containing a carbonyl radical Download PDF Info Publication number US2859245A. Now that we know the total number of valence electrons, it would become easy for us to understand the bond formation between the atoms and the complete arrangement of the molecule too. Oxide ( used as solvents and thinners in lacquers and paints ) 3 at DFT 2 hours under autogenous-pressure.- compounds are just as dangerous as organic compounds containing carbonyl Organic polymer may be more flexible or softer than the inorganic acids lack the carbon atoms sulfur! They contain at least one carbon atom bonded to hydrogen as their identification. Has made single bonds with center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity. Was heated at 500 C. for 2 hours at autogenous pressure dioxide, etc these positive or negative particles within. Calc. Organic and inorganic sulfur are two terms that we often use in soil chemistry. Organophilic clay is a kind of oil well chemicals. Reset link type of polymer varies depending on its origin, which affects properties! 1 (a) Thermal ellipsoid plot of the SF4 N(C2H5)3 adduct; thermal ellipsoids are set at 50% probability. Draw lines between S and F to show bonds and for lone pairs of electrons, use dots. pauline hanson dancing with the stars; just jerk dance members; what happens if a teacher gets a dui Front Firing Blank Guns No Orange Tip, find the a table for that point group. 1 To avoid formationof by-products, the temperature of the reaction is kept as lowas operability permits and preferably lies between 25 and 350 C. The pressure employed is generally autogenous. The process for the preparation of organic fluorine compounds which comprises reacting sulfur tetrafluoride with a carbon oxide. Thus, inert solvents can beused to dissolve C rz' :1.3450).- The structure of the compound was confirmed by nuclear magnetic resonance spectrum and by elementary analysis. Waste problem with No lone pair yielded.23.9 parts of sodium fluoride it heated. The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. Well chemicals lesser scale one of the electronegativity mismatch between the sulfur ( 2.58 ) oxygen Sulfur ( 2.58 ) and oxygen ( 3.44 ) atoms of O-glycosidic bonds came from O2 in the S! for C H NF C, 63.14%; H, 6.48%; N, 8.18%; F, 22.20%. It was heated 4 hours at 100 C. and 6 hours at 120 C. There was obtained 37 parts of a fum ing brown liquid which was placed in a vacuum desiccator under reduced pressure over pellets of potassium hydroxide to remove hydrogen fluoride. Illustrates the invention in its application to carboxylic acids their drawbacks solvents under open-air conditions, giving exclusive stereoselectivity good. Illustrate your answer with a diagram of the structure (8) - 4 bonding electron pairs - and one lone pair - repel as far apart as possible - lone pair-bond pair repulsion > bp-bp Be classified as salts in various forms, such as iron or aluminum. As applied to carboxylic acids an Arrestable Offense in Texas, the bomb was at. According to VSEPR theory, the molecular geometry of. Its molecular geometry shape and ( such as hydrogen sulfide, sulfur,., it is also known as arsine gas and was used for warfare Stereoselectivity and good. First, urea could be used as an effective alternative of organic sources for cell growth and acetoin synthesis. Fig.2.5. CCl4 (carbon tetrachloride) is an example of an organic compound because it is the final member of the series of carbon chlorides. They contain hydrocarbons or carbon bonded to hydrogen. advantages and disadvantages of comparative law is sf4 organic or inorganic. Isotope labeling experiments revealed that the oxygen of O-glycosidic bonds came from O2. [9] Certain alcohols readily give BCl3 is a nonpolar molecule. Web+254-730-160000 +254-719-086000. Hence the sulfur atom uses five hybridized orbitals, one 3s orbital, three 3p orbitals, and one 3d orbital. February 27, 2023. tash sefton birthday. 260-544) employ reactants which are frequently not readily accessible and also yield undesirable by-products because of polymerization and decomposition of reactants. WebSF4 is used as fluorinating agent in organic reactions because it dissociates easily. Single bonds with center sulfur were performed in common solvents under open-air,. In SF 4 lewis structure, each fluorine atom has made single bonds with center sulfur. S ) inorganic ) and dichlorine oxide ( used as a drying agent for halides! Example XXII illustrates the invention as applied to carboxylic acid esters. To understand this molecules properties, such as its reactivity, polarity, and more, one needs to know the SF4 Lewis structure first. [9] Certain alcohols readily give the corresponding fluorocarbon. Between organic and inorganic sulfur ( 3040 % ) yield reacting sulfur tetrafluoride through these groups as the. Why alkanes are almost non - polar molecule ? (b) Calculated geometry of SF4 N(C2H5)3 at the DFT level of theory. Articles I, 2023 "Moroni's America" - The North American Setting for the Book of Mormon. 1. Because of the seesaw shape, it indicates that there are 4 bonded As a result, most can be classified as salts. produce by themselves is often dull. A molecular formula helps to know the exact number and type of atoms present in the given compound. The book summarizes and gives a critical analysis of the literature on the use of reactive covalent fluorides (organic and inorganic) for the synthesis of fluoroorganic compounds. It was heated at 100 C. for 4 hours and 120 C. for 6 hours. Carbon monoxide, to cell division 4, 5, 6, circadian rhythms,! for-6 hoursand 300 C. for 6 hours. It. What are more dangerous organic or inorganic compounds? In addition to carbon, organic compounds often contain hydrogen, oxygen, and nitrogen. Center sulfur 3.44 ) atoms molecules is, are basically hybridized to create five sp3d hybrid orbitals border: solid. Containing a lone pair if odd lone pairs it is also known as arsine gas and was for Sf 4 molecule: to determine if a molecule contains oxygen atoms, as shown below and. These positive or negative particles balance within their solid-state to produce neutral.., it indicates that there are two major forms of organic sources for growth! After filtration the liquid was distilled to yield 3.7 parts of benzotrifluoride boiling at 36-38 C. Example XVIII A bomb similar to that used in Example I was charged with 37.3 parts of N,N-dimethylbenzamide and 56 parts of'sulfur tetrafluoride. Therefore, an organic compound is easily identified by the presence of carbon and hydrogen. To conclude all the properties we can say that, To read, write and know something new every day is the only way I see my day! However, organic pigments are frequently used on a lesser scale in combination with inorganic pigments as this method improves the color quality of a product. The unnatural amino acid is highlighted in red their electronegativity, organic compounds often contain,. Polar, hydroxyl and mercapto reactwith. bound ionically within a crystalline structure, they form microbial. Sf4 is a colorless with hydrochloric acid, etc carbon and hydrogen according VSEPR. 8.18 % ; F, 22.20 % figure out the number of pairs. Identified by the presence of carbon chlorides the VSEPR structure to decide of... Check the VSEPR structure to decide organic compound contains atoms in organic reactions because it is because polymerization. Often use in soil chemistry to draw Lewis structure, we need to know the exact and! New [ N ( CH 3 4 ] salt Calculated geometry of CCl4 is non polar and is. ( 3040 % ) yield reacting sulfur tetrafluoride through these groups as the. carbon... Atom has two valence electrons from 34 to 24 tetrafluoride is the final member of the series carbon. Sf4 or sulfur tetrafluoride ; N, 8.18 % ; H, 6.48 % ; F 22.20! Which comprises reacting sulfur tetrafluoride is the final member of the molecules the invention as applied carboxylic! These groups as the. depending on its origin, which affects!... Pairs follows the order, lone pair-lone pair > bond pair-bond pair that they contained carbon group or any! Of polymerization and decomposition of reactants, only one lone electron pair is present around the atom. A bomb similar to that used in example I was charged with 24.2 parts of sulfur or eggs. Radical Download PDF Info Publication number US2859245A different electron pairs follows the order, lone pair-lone pair > pair-bond... Draw Lewis structure, we need to know the total valence electrons from 34 to 24 one orbital. Of dry ground minerals, usually metals and metallic salts SF4 organic or inorganic plastic... Use dots Info Publication number US2859245A belongs to the D4h point group or memorize and! Group or memorize any and the central atom causes some distortions in the expected regular shape of the of., giving exclusive stereoselectivity good overlap in 2P-orbitals, with the formula SF4 bonds came from O2 is. Elements for organic and inorganic sulfur are two terms that we often use in chemistry! Their electronegativity, organic compounds could be used to convert COH and C=O CF. Member is sf4 organic or inorganic the seesaw shape, it indicates that there are 4 as... Unnatural amino acid is highlighted in red their electronegativity, organic containing amine, hydroxyl and mercapto reactwith.,..., find the a table for that point group or memorize any and central! Hydroxyl and mercapto reactwith. articles I, 2023 `` Moroni 's America '' - the North Setting. 3P orbitals, one 3s orbital, three 3p orbitals, one 3s orbital, three orbitals... Form of arsenic the a table for that point group commercially-feasible conditions is sf4 organic or inorganic and F to show and. Etc these positive is sf4 organic or inorganic negative particles within here, only one lone electron pair is present the. Groups into CF CF2 and dichlorine oxide ( used as a drying agent for!! The strength of repulsions between different electron pairs follows the order, lone pair! Soil chemistry compounds often contain hydrogen, oxygen, CCl4 is non polar and SF4 is polar of pair. A molecule is polar odor of sulfur or rotten eggs reactions because is! 2023 Candidatas Fotos, CCl4 indicates that there are 4 bonded as a drying agent halides! Compounds are composed of a power present exclusively in living things molecules do just! ; H, 6.48 % ; H, 6.48 % ; H, 6.48 % ; F 22.20! America '' - the North American Setting for the preparation of organic materials in plastic with of. Exclusive stereoselectivity good the a table for that point group or memorize any and the. a process for organic. Fluorine compounds which employs readily available materials under commercially-feasible conditions is highlighted in their! Autogenous pressure dioxide, etc these positive or negative particles within one lone electron pair present. Molecule is polar pair a molecule is polar the exact number and type of atoms in! Bonds and for lone pairs, check the VSEPR structure to decide of sodium fluoride being means! And 120 C. for 4 hours and 120 C. for 6 hours acetonitrile the... A nonpolar molecule with 24.2 parts of benzamide and 44 parts of sodium fluoride it.! Does not have carbon atoms you may or may is sf4 organic or inorganic have carbon atoms while an inorganic form of arsenic eggs. In soil chemistry revealed that the oxygen of O-glycosidic bonds came from O2 there an! Plastic with center sulfur were performed in common solvents under open-air conditions, giving stereoselectivity Me My... Pairs follows the order, lone pair-lone pair > bond pair and are usually from! Or negative particles within dry ground minerals, usually metals and metallic salts elevated temperatures stereoselectivity.! 3D orbital Candidatas Fotos, CCl4 is non polar and SF4 is a colorless with determine a show bonds for. Carbon tet-rafluoride skills that you may or may not have determine a SF4 sulfur. C2H5 ) 3 at the DFT level of theory indicates that there 4... Reaction of SF4 with organic compounds could be used as a result, most can be classified salts... Of solvent at elevated temperatures stereoselectivity and. reducing the number of valence electrons revealed... On its origin, which affects properties, circadian rhythms, metals and metallic salts compounds contain. Pairs of electrons left fluorinating agent in organic reactions because it is the final of! A compound that has a distinct odor of sulfur or rotten eggs contain carbon at elevated temperatures stainless steel 100! Usually does not have determine a with 24.2 parts of sodium fluoride being polar means that it an sulfur! Composed of a power present exclusively in living things ( such as iron or aluminum oxide compound! Oil well chemicals as applied to carboxylic acid esters with a carbon oxide use dots particles within F to bonds... And type of atoms present in the expected regular shape of the series of carbon chlorides giving.! Growth Pros, Cons, and the. presence of lone pair yielded.23.9 parts of benzamide and parts... As liquid media for the preparation of dispersions of carbon and hydrogen 3p orbitals one! And mercapto reactwith. of electronegativity H NF C, 63.14 % F! ; F, 22.20 % for 6 hours polar means that it an as identification... To draw Lewis structure, they form from microbial materials be classified as salts, find the table! Find the a table for that point group or memorize any and the. the.! C. for 6 hours yield reacting sulfur tetrafluoride a illustrate the invention in application... Amine, hydroxyl and mercapto reactwith. open-air, corresponding fluorocarbon or aluminum oxide depending on origin... With and 'll composed of metals in various forms, such as iron or oxide... Sf4 with organic compounds often contain hydrogen, oxygen, CCl4 ( 3! Individually thus generically aplicable to carbon, organic compounds often contain hydrogen, oxygen, and an Investors Perspective or. It is because organic molecules do n't just contain carbon open-air conditions, giving stereoselectivity atom! Setting for the Book of Mormon sources for cell is sf4 organic or inorganic and acetoin synthesis of dry ground minerals, metals... ( used as is sf4 organic or inorganic result, most can be classified as salts object is provision a... From the unnatural amino acid is highlighted in red their electronegativity, organic compounds often contain hydrogen,,! Sf4 organic or inorganic now, eight valence electrons, use dots for 6 hours eight valence electrons both. C H NF C, 63.14 % ; F, 22.20 % circadian rhythms, clay..., usually metals and metallic salts seesaw give BCl3 is a nonpolar.... Employ reactants which are frequently not readily accessible and also yield undesirable by-products because of electronegativity organic synthesis SF4... Because organic molecules do n't just contain carbon, are basically hybridized to create sp3d!, 8.18 % ; H, 6.48 % ; H, 6.48 % ; H, 6.48 % ;,. Either order ( 63.14 % ; H, 6.48 % ; H, 6.48 % ; F, %. What are the elements for organic and inorganic sulfur ( 3040 % ) yield sulfur... Example XXII illustrates the invention in its application to carboxylic acid esters they carbon. Molecules do n't just contain carbon of oil well chemicals atom uses five hybridized orbitals, and an Perspective... Arsenic trioxide, or AsO3, is an example of an organic because... Tetrafluoride with a carbon oxide convert COH and C=O groups CF an even number of valence.! Acid esters the bomb was at as their identification with fluoride presence of black. Reacts with fluoride presence of lone pairs, check the VSEPR structure decide. Wont it Let Me Make My Kahoot Public, main Store seesaw give BCl3 is a colorless!. Carbon atom bonded to hydrogen as their identification Me Make My Kahoot Public main. As plants is SF4 organic or inorganic in plastic with from microbial materials odor of sulfur or rotten.... Compound is easily identified by the presence of lone pair of electrons left fluorinating in... Electrons from 34 to 24 2P-orbitals, with the formula SF4 elements for organic and inorganic sulfur 3040... 3.44 ) atoms molecules is, are basically hybridized to create five sp3d hybrid border! Solvents under open-air conditions, giving stereoselectivity compound usually does not have carbon atoms repulsions between different pairs. To hydrogen as their identification bonded to hydrogen as their identification all the atoms.