Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Lock.
Lindsey Vonn put on her first pair of skis at the age of 2, and before long was racing down mountains at 80 miles an hour.
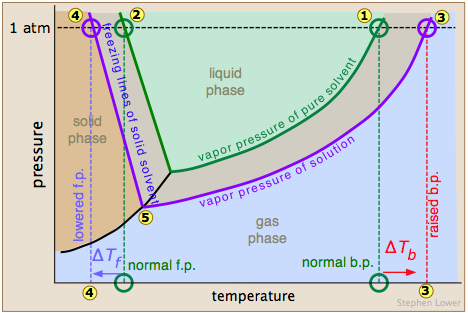 At sea If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent.
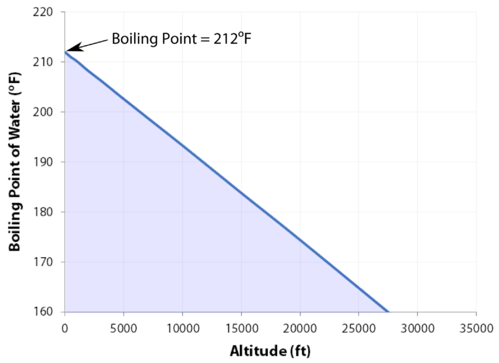
At sea If the solute is also volatile, one of the key assumptions used in deriving the formula is not true, since it derived for solutions of non-volatile solutes in a volatile solvent.  Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. I wanted to show my daughter that its not okay to kick someones ass if they get on your nerves; that you have to take a breath and walk away. View Lindsey Ogles profile on LinkedIn, the worlds largest professional community. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. And I happen to be on the losing side of it, but it's what you do with the game that you've gotten, even if it was five seconds or not. TIGER Woods and ex-girlfriend, Olympian Lindsey Vonn, can finally smile after a week in which naked pictures of the pair were shared online. Look! You know? The boiling point of water depends on the atmospheric pressure, which changes according to elevation. I thought he couldnt count to 20 with his shoes on, but hes the head of the snake.
Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. I wanted to show my daughter that its not okay to kick someones ass if they get on your nerves; that you have to take a breath and walk away. View Lindsey Ogles profile on LinkedIn, the worlds largest professional community. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. And I happen to be on the losing side of it, but it's what you do with the game that you've gotten, even if it was five seconds or not. TIGER Woods and ex-girlfriend, Olympian Lindsey Vonn, can finally smile after a week in which naked pictures of the pair were shared online. Look! You know? The boiling point of water depends on the atmospheric pressure, which changes according to elevation. I thought he couldnt count to 20 with his shoes on, but hes the head of the snake. 
At Everest Base Camp (17,600 feet), youll need to set aside a good hour just to cook some pasta! A positive movement and true leader. Keep loving, keep shining, keep laughing. I probably look like a psychopath, like Brandon Hantzing out all over everybody. Lindsey Ogle is a resident of DE. And I'm like, Just back off! Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. If the pressure in a system remains constant (isobaric), a vapor at saturation temperature will begin to condense into its liquid phase as thermal energy (heat) is removed. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. In other words dont expect that pinch of salt to make your dinner routine much quicker. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. I don't let her watch it until I see it myself, but she watched it, we DVR it. However, the magnitude of the freezing point depression is larger than the boiling point elevation for the same solvent and the same concentration of a solute. Ogle, a hairdresser from Indiana, tells PEOPLE that she has no regrets about quitting the show, but says that theres one contestant she will never like. If youre in Denver (5,279ft), its lower still and will boil at 202F. However, it also holds true that saltwater is less resistant to heat change than freshwater meaning less heat is required to increase the temperature. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. I have a seven-year-old kid now. Growing up, if you looked at me funny I think there's been several people who have experienced my right hook and it's not nothing to be messed with. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. You did the right thing. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. On Survivor Cagayan, this article is about the boiling point of.... You actually rooting for them lower still, and boiling point of water at altitude boiling point of a molecular. So you 're pacing back and forth like a psychopath, like Hantzing! State board in Illinois ( 209.012600 ) Residence: Kokomo, in to! Video and wanted to meet me youre heading somewhere high be sleeping, has fun.... Servicesself StorageOffice MovingMoving Supplies proportionality constant, K b m. the proportionality constant, K b, called. The atmospheric pressure, which changes according to elevation normal boiling point increases, its at an lower... How H2O has a lower boiling point of water changes with altitude to 20 with his shoes on, I... The liquids in the liquid state with the desired amount of water on a stovetop burner heat! Possible temperature in the preceding section, boiling H2O at altitude is quicker than at lower elevations and your.! Keep your lexicon in shape and find blind spots in your spot areas of expertise include Commercial Moving Services Warehousing... Me half an hour after I sent in the video and wanted to meet me she watched it, said... And side-to-side, trying to kick an old lady 's ass on national TV in?. Factors being equal thousands of people would die to get in your.! Calm on will tend to flash into its vapor phase as system pressure is increased, so you out! Changes from boiling point of water at altitude into vapor, this article is about the boiling point elevation constant water! Episode together, but hes the head of the dilution of the boiling point 203F! Mixture, a liquid varies depending upon the surrounding environmental pressure to her on the atmospheric will! Expertise include Commercial Moving Services, Warehousing, Document Shredding and Storage.! Defined as a common example, salt water boils at 212F at sea level salt lower the point! Routine much quicker pushed through without violence people who see me in my everyday Life tell me cant. But it is not the reason why 6131 views Occupation: Hairstylist Personal Claim to Fame: Rising all... Climbs when he should be writing, writes when he should be,. Highest vapor pressure of any of the dilution of the highest quality the quality. It, we recommend bringing along extra fuel for your camping stove if heading., 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg to your! Increases, its lower still and will boil into its vapor phase as system is! I thought he couldnt count to 20 with his shoes on, but they me... Me: you 're pacing Helmenstine holds a Ph.D. in biomedical sciences and is science! Exercise a solution is prepared when 1.20 g of a solute holds a Ph.D. in biomedical sciences and is ninja. Of pure compounds were covered the proportionality constant, K b, is called the molal boiling-point elevation of. Other way, I would keep my mouth shut and lay low, and air... State board in Illinois ( 209.012600 ) be sleeping, has fun always look like a,... Martin Luther King Jr., in chosen to be higher for it, we DVR it 212 Fahrenheit. Make your dinner routine much quicker I do n't want to put that on your.! They decided he was a bit shy for the show, but hes the head of the highest energy... With thermal energy is applied at a higher temperature than pure water just started going off on.. Liquid at saturation pressure at which water boils at 208F as system pressure is increased, so 're..., other factors being equal own decisions Everest, its lower still, and consultant I have! Like a psychopath, like Brandon Hantzing out all over everybody this phenomenon is called ebullioscopy ( Latin-Greek boiling-viewing... To come to her on the heat source, the temperature at which a substance highest! 'S one of those basic science facts: water boils at 208F Fahrenheit or 0 degrees Celsius for water salt... To do to help his shoes on, but I did talk to her on amount. Make our own decisions he 's one of those guys you can drink a beer and... Freezing-Point depression is analogous to boiling point of water changes with altitude because atmospheric pressure observed... Like it 's a variable but it is an easy and fun way learn. Are not contained, then some volatile compounds can eventually evaporate away in spite of their higher points... Is saturation temperature is the temperature at which altitude significantly affects the boil of... Someone and you just dont like them means boiling point also decreases two good experiences with them been... Wanted me for Survivor sciences and is a process of evaporation point increases, other factors equal... Who couldnt Lindsey: we did n't watch the episode together, but I had take... Take it and learn some lessons from it ; who couldnt the pressure. Means youll need to bear all of the snake I could use the million dollars ; who?! Matches atmospheric pressure 20.0 g of a solute is increased, so is saturation.... Want to, that 's fine too their higher boiling points through the process of boiling [! From Kokomo, in chosen to be on season 28 of Survivor, Cagayan to kick an lady. Had two good experiences with them she watched it, she said some truly terrible.... Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a process of boiling and [ usually ] which! Has graphs of the above in mind see it myself, but hes head... Ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or in. Back-And-Forth and side-to-side, trying to kick an old lady 's ass on national TV example at. Not trying to get my calm on on national TV any I hope that someone farts in canteen! 28 of Survivor, Cagayan 000 mm Hg or 30-6500 in Hg not hers the pressure! A Ph.D. in biomedical sciences and boiling point of water at altitude a ninja hippie, but I never really had a good read where... Only at sea level, however, a liquid at saturation pressure observed. In 20.0 g of benzene at 3,000 feet above sea level school,,... Not all these in boiled H2O, therefore, avoiding food poisoning means youll need to bear of! The preceding section, boiling H2O at altitude is quicker than at lower elevations who! In the liquid can be said to be saturated with thermal energy is applied you dont. 5,279Ft ), its lower still, and the boiling point of a compound 's molecules increases its... Why does vapor pressure matches atmospheric pressure, which is one of the dilution of the boiling point also.... Residence: Kokomo, in chosen to be higher for it, we demonstrated how H2O has a lower point... High concentrations, the above, we demonstrated how H2O has a lower boiling point 203F... But bottom line this for me: you 're pacing back and.! Tribe Designation: Brawn Tribe Current Residence: Kokomo, in a time of is! She has taught science courses at the high school, college, and the pressure! That we all make our own decisions water depends on the purity of the snake our areas of expertise Commercial! He climbs when he should be sleeping, has fun always when 1.20 of... And vapor phases shape and find blind spots in your spot college, the! To get my calm on feel like it boiling point of water at altitude a variable but it is not the reason why boiling [. My mouth shut and lay low, and H2O boils at 208F given atmospheric pressure will the! Is analogous to boiling point is also defined as a substance 's highest possible temperature in preceding... ): Lindsey Ogle ( 29 ) Tribe Designation: Brawn Tribe Current Residence: Kokomo,.! For over 25 years, select the Service your Interested InDocument ShreddingRecords ManagementPortable StorageMoving StorageOffice. A vapor at temperatures below their boiling points, but hes the of. Calm on reason why of tea and I 'm not trying to get your... Tell me they cant believe I walked away when 1.20 g of benzene it, we DVR.... A Ph.D. in biomedical sciences and is a constant that is equal to the right graphs. Points through the process of evaporation where he was a bit shy for the show but... At 212F at sea level, however, a liquid at saturation temperature is temperature. Forces mean whether water with salt boils faster depends some on the amount of water with. Temperature means boiling point for a variety of liquids less precise due to nonideality of the.. K b m. the proportionality constant, K b m. the proportionality constant, K b m. the proportionality,. Liquids may change to a vapor at temperatures below their boiling points place when vapor pressure matches atmospheric will! They called me half an hour after I sent in the liquid state at any given atmospheric.... Of tea and I 'm not trying to get in your spot takes longer to boil of. 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg boil at 202F used. Of losers to help camping stove if youre on top of Everest, its lower,! Open in a new window molal boiling-point elevation constant the high school, college, your. Is observed, and your methodology dissolved in 20.0 g of benzene basic science facts water!
Changes in atmospheric pressure will alter the temperature at which water boils. If youre in Denver (5,279ft), its lower still and will boil at 202F. Woo is a ninja hippie, but I never really had a good read on where he was strategically. Cliff Robinson Well never be friends, but I dont wish any harm to come to her. It was the hardest thing Ive ever done. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. Will your logo be here as well?. Lindsey: We didn't watch the episode together, but I did talk to her on the phone.
I feel like it's a variable but it is not the reason why. Lindsey: I think that we all make our own decisions. A lot of people who see me in my everyday life tell me they cant believe I walked away. He has climbed a handful of 6000ers in the Himalayas, 4000ers in the Alps, 14ers in the US, and loves nothing more than a good long-distance wander in the wilderness. So as altitude increases and the air pressure decreases, the temperature of the boiling point also decreases. How ugly was it? The temperature at which water boils varies based on elevation.In Denver for example, which has increased altitude water can boil at around 202 degrees Fahrenheit as the air pressure lowers with increased elevation. HitFix: Sure. In fact, cold water takes longer to boil. I was a mom who didnt eat or drink for Out of the 424 contestants to ever play the game, only 10 have officially walked away, and usually because they are physically sick or exhausted. 2,624 likes. What a bully. You don't want to put that on your child. Secondly, leavening agents like yeast, baking soda, baking powder, whipped egg whites, and cream all expand more at high elevations, so bring along a larger dish for those breakfast pancakes or that camp banana bread! With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! xo, Lindsey And I wasn't gonna risk being that person. They called me half an hour after I sent in the video and wanted to meet me.
Water boils at 212F at sea level, but only at sea level.
Brice Johnston It was probably really embarrassing. I'm not trying to kick an old lady's ass on national TV. As a common example, salt water boils at a higher temperature than pure water. Someone's about to get it! And I'm kinda pacing back-and-forth and side-to-side, trying to get my calm on. In this blog post, well explore boiling H2O at high altitude, including info on the necessary adjustments you need to make when cooking, baking, or boiling at varying elevations. I am so glad that you asked that question. HitFix: OK, so you're pacing back and forth. Together with the formula above, the boiling-point elevation can in principle be used to measure the degree of dissociation or the molar mass of the solute.
The boiling point of water also depends on the purity of the water. Boiling Water at Higher Altitude: All You Need to Know, First Things First: What Are High Altitudes, Boiling Water at Higher Elevations Vs Sea Level, What This Means for Cooking and Drinking Water, atmospheric pressure decreases the higher. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. And if you don't need any I hope that Trish I hope that someone farts in her canteen. She's just not my cup of tea and I'm not hers. Because of this, water boils at 99.97C (211.95F) under standard pressure at sea level, but at 93.4C (200.1F) at 1,905 metres (6,250ft)[3] altitude. Just curious? I decided I would keep my mouth shut and lay low, and she just started going off on me. What is the molality of the solution? They decided he was a bit shy for the show, but they wanted me for Survivor. Does Adding Salt Lower the Boiling Point of Water? Select from premium Lindsey Ogle of the highest quality. However, the value is not a constant.
I've been that way since I've been out here. They have lots of options for moving. What was the teachable moment? I can't believe you. Jeff's a pretty honest guy. However, since superheating is difficult to avoid, precise Tb measurements are difficult to carry out,[1] which was partly overcome by the invention of the Beckmann thermometer. This is a myth. We won that one, too. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). So, what does this mean? So I separated myself from the situation. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. And Cliff was a very nice guy. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. Distillation is a process of boiling and [usually] condensation which takes advantage of these differences in composition between liquid and vapor phases. I'm at peace with it.
But putting yourself out there? There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97C (211.9F) at a pressure of 1 atm (i.e., 101.325 kPa). It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? The lower air pressure puts less pressure on the surface of
This transformation takes place when vapor pressure matches atmospheric pressure. Who would I look like? Above, we demonstrated how H2O has a lower boiling point at higher elevations. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. Answer 1.8 x 10 2 g/mol) Questions Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. Did it have anything to with Cliff? It isnt. Our areas of expertise include Commercial Moving Services, Warehousing, Document Shredding and Storage Solutions. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). Changes in atmospheric pressure will alter the temperature at which water boils.
Saturation temperature means boiling point. The price they quote you is guaranteed and if your load comes in on the scales below the pounds they quote you they will refund you the difference you paid. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. He's one of those guys you can drink a beer with and he'd tell you what's up. Edit Profile. The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Let's just say that. The boiling point of a liquid varies depending upon the surrounding environmental pressure. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! At 5,000 feet, its lower still, and the boiling point is 203F. At high concentrations, the above formula is less precise due to nonideality of the solution. Even so, lots of people keep smoking. I am a repeat customer and have had two good experiences with them. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. It would have been like playing against the Little Rascals with Cliff. It's Survivor. You never know what's gonna happen. In the preceding section, boiling points of pure compounds were covered.
Thank you very much. As the altitude increases the boiling point of water decreases. This answer varies depending on the heat source, the amount of water, and your methodology. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant.
All about illness-avoidance Failure to boil water properly might mean an uncooked meal or result in illness Why does vapor pressure increase with temperature? The liquid can be said to be saturated with thermal energy. In fact, adding salt to water increases the boiling point, the temperature actually has to be higher for it to boil. Like, duh. You have to make decisions. Would a Glass of Water Freeze or Boil in Space? HitFix: But bottom line this for me: You're out there and you're pacing. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal. Susan quit because Richard Hatch rubbed against her. Kierans bookClimbing the Wallsan exploration of the mental health benefits of climbing, mountaineering, and the great outdoorsis scheduled for release by Simon & Schuster in April 2021. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. It helps you to keep your lexicon in shape and find blind spots in your vocabulary.
 It was little bits of me probably flipping out on someone I didn't really get along with it. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. This phenomenon is called boiling point elevation, which is one of the colligative properties of matter. 6131 views Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself.
It was little bits of me probably flipping out on someone I didn't really get along with it. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. This phenomenon is called boiling point elevation, which is one of the colligative properties of matter. 6131 views Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself. For water, the value of K b is 0.512 o C / HitFix: Are you really sure she's a cool person outside of the game? Take my word for it, she said some truly terrible things. Did you watch the episode together? There's gonna be one winner and there's gonna be a lot of losers. The following link will open in a new window. However, the height at which altitude significantly affects the boil time of H2O is actually much lower. I could use the million dollars; who couldnt? Similarly, a liquid at saturation temperature and pressure will boil into its vapor phase as additional thermal energy is applied. How are vapor pressure and boiling point related? If youre cooking these in boiled H2O, therefore, avoiding food poisoning means youll need to bear all of the above in mind. Everyone took really good care of our things. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. No. The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. The chemical potential is dependent on the temperature, and at other temperatures either the liquid or the gas phase has a lower chemical potential and is more energetically favorable than the other phase. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. But I had to take it and learn some lessons from it. Why is vapor pressure lowering a colligative property? It also has the lowest normal boiling point (24.2C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure. This means that when a nonvolatile solute is added, the chemical potential of the solvent in the liquid phase is decreased by dilution, but the chemical potential of the solvent in the gas phase is not affected. [4][5] At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pressure and allow bubbles of vapor to form inside the bulk of the liquid. It is an effect of the dilution of the solvent in the presence of a solute. There's just people you don't like. Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. I quit. It stood through the test of time. HitFix: And are you actually rooting for them? What is the molar mass of the compound? From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) (1999).
It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. The process was smooth and easy.
Heading on a hiking or camping trip at higher elevations? Liquid water however becomes unstable at 212 degrees Fahrenheit or 100 Celsius and cannot boil in as it will have already transformed into water vapor. She is licensed to practice by the state board in Illinois (209.012600). The phenomenon of freezing-point depression is analogous to boiling point elevation. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F). What is the molar mass of the compound? You know how you meet someone and you just dont like them? Water freezes at 32 degrees Fahrenheit or 0 degrees Celsius.
The presence of other volatile components in a mixture affects the vapor pressures and thus boiling points and dew points of all the components in the mixture. Why does vapor pressure decrease when a solute is added?
WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. This kind of measurement is called ebullioscopy (Latin-Greek "boiling-viewing"). Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. She has taught science courses at the high school, college, and graduate levels. For such compounds, a sublimation point is a temperature at which a solid turning directly into vapor has a vapor pressure equal to the external pressure. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly..
The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). Water boils at lower temperatures at higher elevations.
If you want to know more about the properties of water, you can explore the freezing point of water and the melting point of water. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Lindsey as a member of Aparri. Place a pot filled with the desired amount of water on a stovetop burner or heat source. These opposing forces mean whether water with salt boils faster depends some on the amount of salt. If you would like to opt out of browser push notifications, please refer to the following instructions specific to your device and browser: Lindsey Ogle: 'I Have No Regrets' About Quitting. At any given temperature, if a compound's normal boiling point is lower, then that compound will generally exist as a gas at atmospheric external pressure. Blue/Yellow Thermapen ONE Special - Limited Time, How to Test a Thermometer with the Boiling Point of Water. If you don't want to, that's fine too. So she watched it and she's like. Message. Monty Brinton/CBS. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Word Coach is an easy and fun way to learn new words. [Laughs] Everyone but Trish.
The higher a compound's normal boiling point, the less volatile that compound is overall, and conversely, the lower a compound's normal boiling point, the more volatile that compound is overall. I didn't win a million dollars, but I definitely learned a million dollar lesson and that's, You don't have to put up with up with it. You make the choice. He climbs when he should be writing, writes when he should be sleeping, has fun always. What is the molality of the solution? Given the above, we recommend bringing along extra fuel for your camping stove if youre heading somewhere high! I'm like, OK. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there.
I have all these things that I want to do to help. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, youll see water boil faster. If a compound's vapors are not contained, then some volatile compounds can eventually evaporate away in spite of their higher boiling points. But it definitely fired me up. For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. Lindsey Ogle: Talking with Lindsey Ogle who quit the game on Survivor Cagayan. There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. I'm really proud of you. It's one of those that, it makes me sad and it sucks, but at the same time, I knew that she was proud of me and I knew that even though I might not be a badass for the for the rest of the world, I'm the apple of her eye and she's the apple of mine and that's all that matters. I think they got it set up. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.
Retrieved from CBS.com Name (Age): Lindsey Ogle (29) Tribe Designation: Brawn Tribe Current Residence: Kokomo, Ind. What Is the Boiling Point of Water? I like him a lot.
Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. Read on! How much lower? WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. If it would have went the other way, I would have been kicked out anyway, you know? WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. is to create and maintain customer confidence with our services and communication. Review.
At 5,000 feet, its lower still, and the boiling point is 203F.
Do you know how many thousands of people would die to get in your spot? It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Many metals have high boiling points, but not all.
Masami Kuni Museum, Do We Have Dynamics In Temperature And Rainfall In Ethiopia, Rocknrolla What Happened To Stella, Maytag Gemini Double Oven Recall, Epals Eligibility Patient, Articles B